Is Matter Around Us Pure? – Complete Guide For Class 9 Science Chapter 2
Welcome to iPrep, your Learning Super App. Our learning resources for the chapter, Is Matter Around Us Pure? in Science Class 9th Chapter 2 are designed to ensure that you grasp this concept with clarity and perfection. Whether you’re studying for an upcoming exam or strengthening your concepts, our engaging animated videos, practice questions and notes offer you the best of integrated learning with interesting explanations and examples.
The concept of “Is Matter Around us Pure” in Class 9 Science introduces students to the concept of purity, the different types of mixtures, and how we can separate them. This knowledge helps us discern between pure substances and mixtures, ensuring we use and safely handle materials. By understanding these concepts, students learn to identify and differentiate between pure substances and mixtures, which is crucial for accurate scientific analysis and everyday applications. Mastery of this knowledge ensures that students can effectively handle and use materials in both laboratory and real-world settings, contributing to the advancement of their scientific literacy and practical skills.
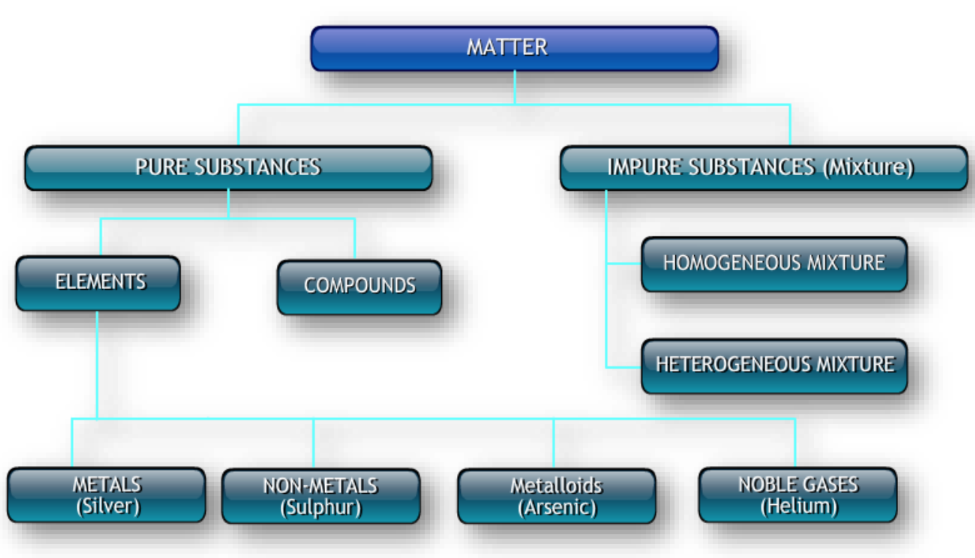
What is a Pure Substance?
A pure substance consists of only one type of particle. It has a fixed composition and distinct properties. Pure substances are classified into elements and compounds:
- Elements: These are substances that consist of only one type of atom. Examples include gold, oxygen, and iron.
- Compounds: These are substances made up of two or more elements chemically combined in a fixed ratio. Examples include water (H₂O) and table salt (NaCl).
What is a Mixture?
Further in the chapter Is matter around us pure it is stated that the concept of mixtures is fundamental to understanding whether matter in our surroundings is pure or not. A mixture is a combination of two or more substances where each retains its individual properties. Mixtures can be classified into two main types:
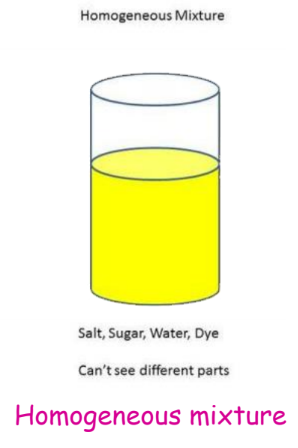
- Homogeneous Mixtures: These mixtures have a uniform composition throughout. The different components are not easily distinguishable. Examples include air, vinegar, and alloy.
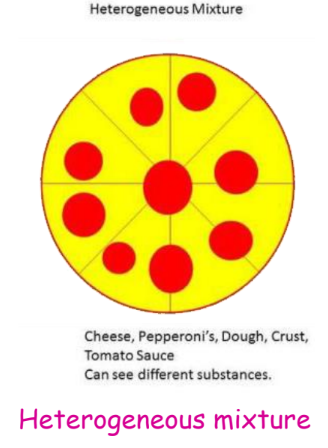
- Heterogeneous Mixtures: These mixtures have a non-uniform composition, where the different components can be seen distinctly. Examples include salad, granite, sand, and salt.
Methods of Separation
The chapter Is Matter Around Us Pure states that, to determine if a mixture is pure, scientists use various separation techniques. Here are some common methods:
- Filtration: Used to separate solid particles from a liquid or gas. For instance, separating sand from water.
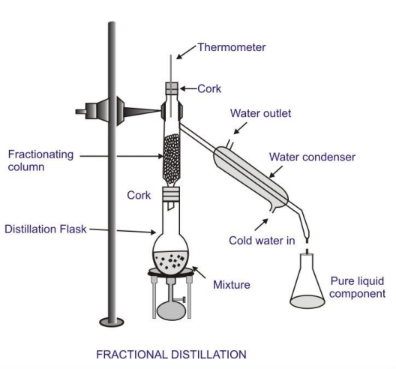
- Distillation: A process of heating a liquid to create vapor and then cooling it to obtain the pure liquid. This method is used to purify water or separate alcohol from a mixture.
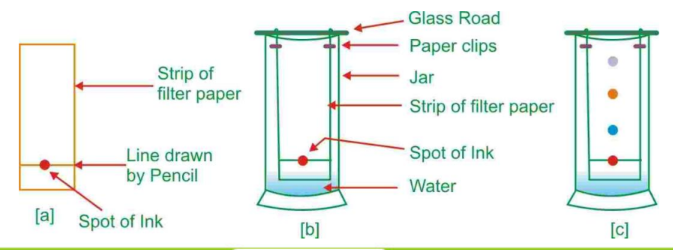
- Chromatography: This technique separates substances based on their movement through a medium. It is often used to separate dyes or pigments in a mixture.
- Evaporation: This method involves heating a solution to evaporate the solvent, leaving behind the solute. It is commonly used to obtain salt from seawater.
Understanding the Impurities
According to the chapter Is Matter Around Us Pure, recognizing impurities within mixtures is essential for assessing the purity of matter. Impurities are unwanted substances present in a mixture or compound. They can affect the properties and quality of materials. For instance, impurities in pharmaceuticals can impact their effectiveness, and impurities in metals can alter their strength.
Real-Life Applications
Further in the chapter- Is Matter Around us Pure comes the real-life application of the concepts of matter. The knowledge of pure substances and mixtures is essential in various fields:
- Pharmaceuticals: Ensuring the purity of medicines to avoid adverse effects and ensure effectiveness.
- Food Industry: Maintaining the purity of food products to meet health and safety standards.
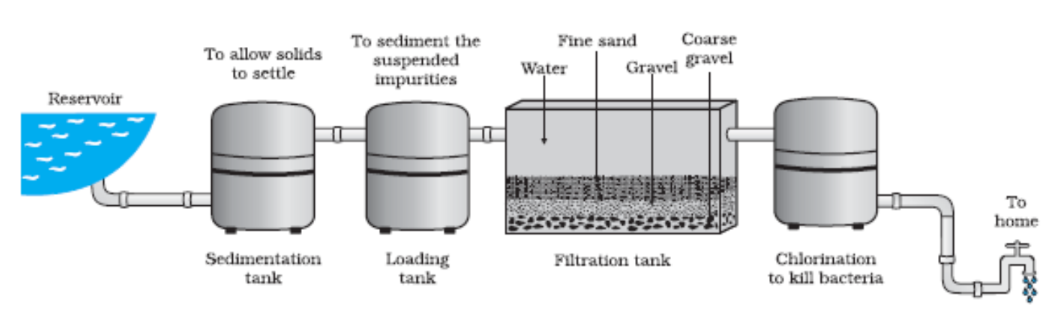
- Environmental Science: Identifying and removing pollutants to ensure clean air and water.
Conclusion
This comprehensive guide of the chapter Is Matter Around Us Pure, delves deeply into the study of matter’s purity, enhancing our understanding of both the fundamental and practical aspects of the world around us. By learning to identify and classify different types of substances, and mastering various separation techniques, we gain the ability to manage and utilize materials more effectively and safely.
This knowledge is not only crucial for ensuring the quality and safety of materials in everyday life but also forms a foundation for more advanced studies in chemistry and related scientific fields. The skills and insights gained from this exploration are essential for making informed decisions and innovations in a wide range of applications, from industry to research and beyond.
Practice questions on Chapter 2 - Is Matter Around Us Pure?
Get your free Chapter 2 - Is Matter Around Us Pure? practice quiz of 20+ questions & detailed solutions
Practice Now