Atoms and Molecules – Complete Guide For Class 9 Science Chapter 3
Welcome to iPrep, your Learning Super App. Our learning resources for the chapter, Atoms and Molecules in Science Class 9th chapter 3 are designed to ensure that you grasp this concept with clarity and perfection. Whether you’re studying for an upcoming exam or strengthening your concepts, our engaging animated videos, practice questions and notes offer you the best of integrated learning with interesting explanations and examples.
The concept of “Atoms and Molecules” in Class 9 Science introduces students to the fundamental building blocks of matter, explaining the basic units that constitute all substances. It explores how atoms, as the smallest indivisible units of elements, combine to form molecules, which are the building blocks of compounds. This foundational knowledge is essential for understanding chemical reactions, bonding, and the structure of matter, providing a critical basis for further study in chemistry and its practical applications in the real world.
Atoms and Molecules: The Building Blocks of Matter
The chapter “Atoms and Molecules,” a fundamental part of Class 9 Science, unveils the basic building blocks of matter. This essential topic introduces students to the concepts of atoms and molecules, explaining how these tiny particles form the foundation of everything we encounter in our daily lives.
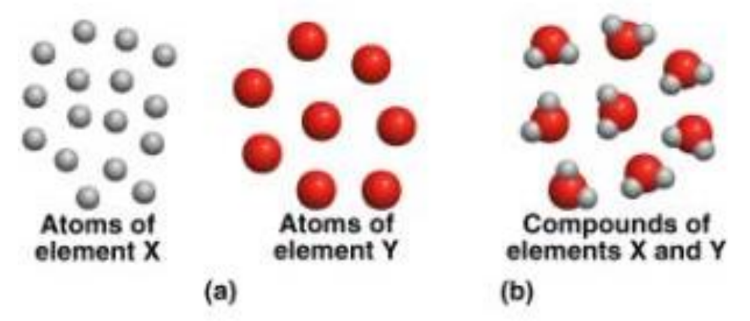
What Are Atoms?
Atoms are the smallest units of matter that retain the properties of an element. They are the building blocks from which all matter is constructed. Each atom consists of a nucleus, containing positively charged protons and uncharged neutrons, surrounded by negatively charged electrons. The unique arrangement and number of these subatomic particles determine the properties of each element.
What Are Molecules?
Molecules are formed when two or more atoms bond together chemically. These bonds can be simple, such as in a molecule of hydrogen gas (H₂), or complex, involving various atoms, as seen in organic compounds like glucose (C₆H₁₂O₆). Molecules are responsible for the diversity of substances and their properties, from the air we breathe to the food we eat.
The Importance of Chemical Formulas
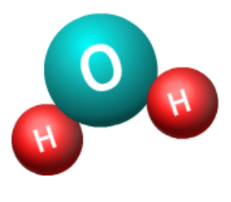
Chemical formulas are symbolic representations that describe the composition of molecules. They provide insight into the types and numbers of atoms in a molecule. For example, the formula H₂O indicates a water molecule with two hydrogen atoms and one oxygen atom. Understanding these formulas is crucial for comprehending chemical reactions and the interactions between different substances.
Atoms and Molecules in Everyday Life
Atoms and molecules play a vital role in all aspects of life. From the air we breathe to the food we consume, everything is made up of these fundamental particles. Knowing how atoms and molecules interact helps us understand chemical reactions, material properties, and the processes that drive the physical and biological world.
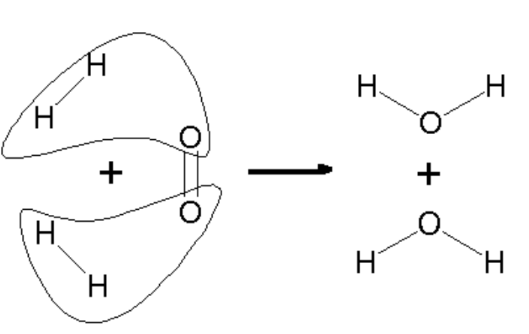
Chemical Reactions and Bonding
The chapter also explores how atoms and molecules interact through chemical bonding. Chemical reactions occur when bonds between atoms are broken and new bonds are formed, leading to the creation of new substances. This understanding is essential for grasping how materials change and transform in various contexts.
Applications and Implications
The study of atoms and molecules is not just academic; it has practical implications in fields such as medicine, environmental science, and technology. For instance, understanding molecular structures aids in drug development, while knowledge of chemical reactions is crucial for industrial processes.
This comprehensive guide on “Atoms and Molecules” offers an in-depth exploration of the fundamental building blocks of matter, detailing their roles in the structure and properties of substances. It delves into the nature of atoms as the smallest units of elements and how they combine to form molecules, which are the fundamental entities of chemical compounds. By understanding these concepts, students gain insights into the nature of chemical reactions, bonding, and the diverse applications of chemistry in real-world scenarios. This foundational knowledge is crucial for advancing the study of science and for applying these principles in various scientific and practical contexts.
In conclusion, Class 9 Science Chapter 3 – Atoms and Molecules lays the foundation for understanding the intricate structure of matter. This chapter not only explains how atoms and molecules form the building blocks of everything around us but also paves the way for comprehending more complex topics in chemistry. Through the study of atoms and molecules, students can unlock the mysteries of chemical reactions, bonding, and molecular structures, which are essential for both academic progress and real-world applications.
At iPrep, we believe that mastering Class 9 Science Chapter 3 – Atoms and Molecules is key to excelling in chemistry. Our engaging resources, including animated videos, practice questions, and notes, are designed to make learning this chapter both enjoyable and effective. As you explore atoms and molecules, remember, in the words of Carl Sagan, “The beauty of a living thing is not the atoms that go into it, but the way those atoms are put together.”
With iPrep’s guidance, you’re on the right path to not only understanding but also appreciating the fascinating world of atoms and molecules. Keep learning, and let the power of science transform your understanding!
Practice questions on Chapter 3 - Atoms and Molecules
Get your free Chapter 3 - Atoms and Molecules practice quiz of 20+ questions & detailed solutions
Practice Now








