Equilibrium – Complete Guide for Class 11 Chemistry Chapter 6
Welcome to iPrep, your Learning Super App. Our learning resources for Chapter 19, “Equilibrium,” in Class 11 Chemistry are meticulously designed to ensure students gain a comprehensive understanding of this essential topic. These resources include detailed notes on concepts such as chemical equilibrium, dynamic equilibrium, and the factors affecting equilibrium. They provide in-depth explanations of the laws governing equilibrium, including Le Chatelier’s principle and the equilibrium constant.
Visual aids, such as diagrams and flowcharts, are used to help students visualize complex processes. Interactive quizzes and practice problems are also included to test comprehension and reinforce learning. Additionally, our resources offer real-life examples and applications to illustrate the relevance of equilibrium in biological systems. These materials are curated to facilitate a deep and thorough grasp of equilibrium for Class 11 students.
This chapter examines how organisms maintain homeostasis through various feedback mechanisms and physiological processes. It covers topics like chemical equilibrium in cells, the role of enzymes in maintaining balance, and the importance of equilibrium in metabolic pathways. The chapter also explores how external factors, such as temperature and pH, influence equilibrium states in living organisms. By understanding these concepts, students gain insights into the dynamic nature of biological systems and how they adapt to changing environments.
The Concept of Equilibrium
Equilibrium, in the context of Class 11 Chemistry Chapter 6: Equilibrium, refers to a state in a chemical system where the rates of the forward and reverse processes are equal, resulting in a stable concentration of reactants and products. This dynamic balance allows systems to maintain consistency despite ongoing changes. In chemistry, equilibrium often involves reversible reactions, where substances can transform back and forth between reactants and products. In biological systems, equilibrium plays a crucial role in maintaining homeostasis, enabling organisms to regulate internal conditions despite external fluctuations. Understanding equilibrium is fundamental to grasping various concepts in chemistry and biology, as it underpins many essential processes, from metabolic reactions to ecological interactions.
In chemistry, most reactions are reversible, meaning they can proceed in both directions.
Reversible Reactions
A reversible reaction is a process that can move forward and backward simultaneously.
Examples of Reversible Reactions:
- Nitrogen and Hydrogen: N2(g)+3H2(g)⇌2NH3(g)
- Carbon Monoxide and Hydrogen: CO(g)+2H2(g)⇌CH3OH(g)
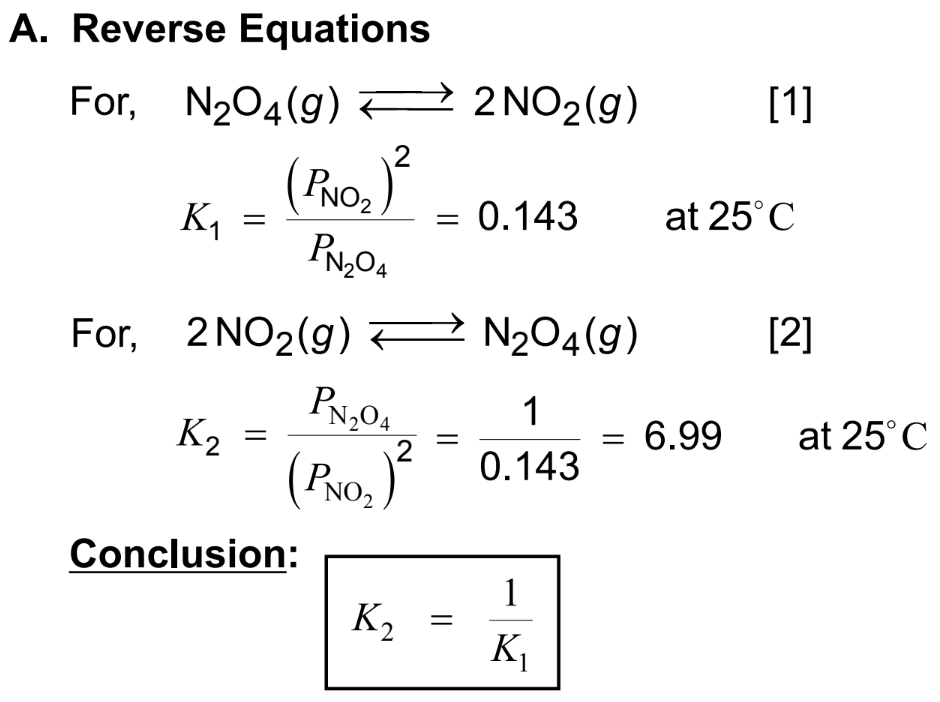
- Dinitrogen Tetroxide and Nitrogen Dioxide: N2O4(g)⇌2NO2(g)
Double arrows (⇌) denote an equilibrium reaction, indicating that the reaction can proceed in both directions.
Chemical Equilibrium
Consider the reaction:
N2O4(g)⇌2NO2(g)
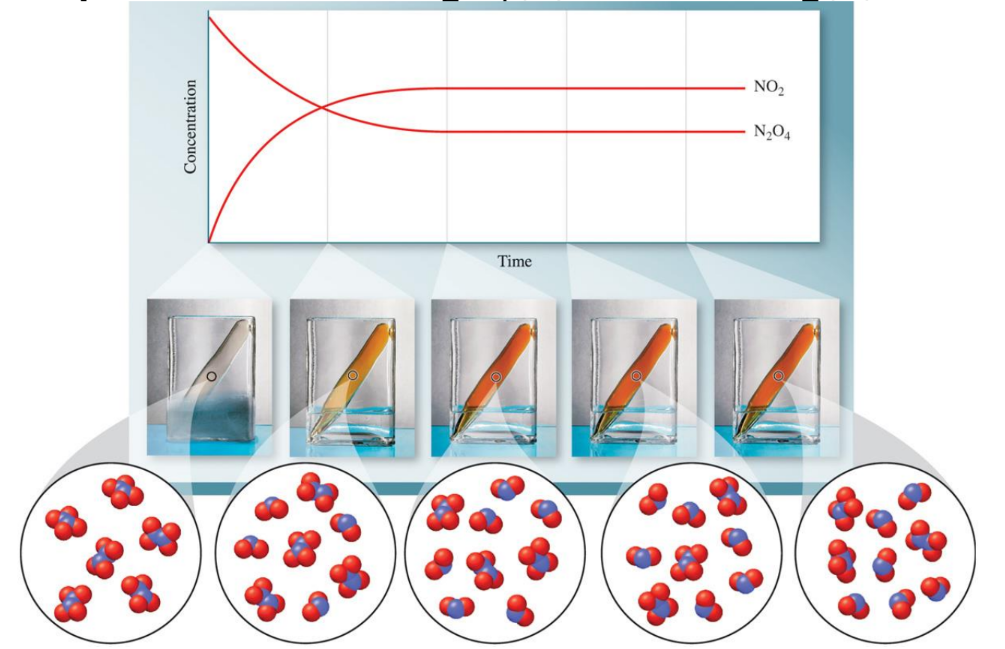
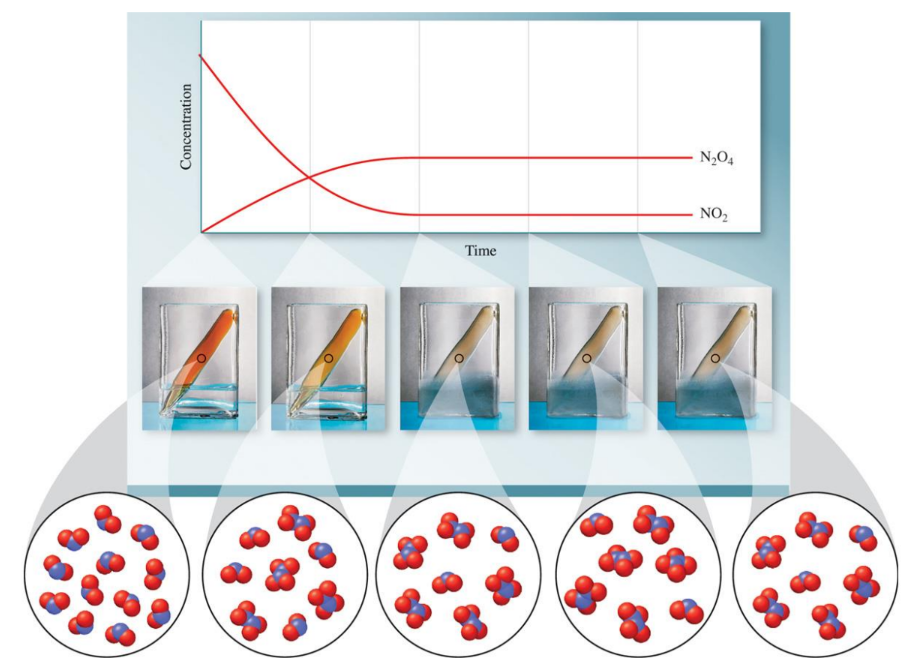
At equilibrium:
- The forward reaction: N2O4(g)→2NO2(g)
- The reverse reaction: 2NO2(g)→N2O4(g)2
Both reactions proceed at equal rates. This state is dynamic, meaning the reactions do not stop; instead, they continue to occur at the same rate in both directions, maintaining the concentration of reactants and products.
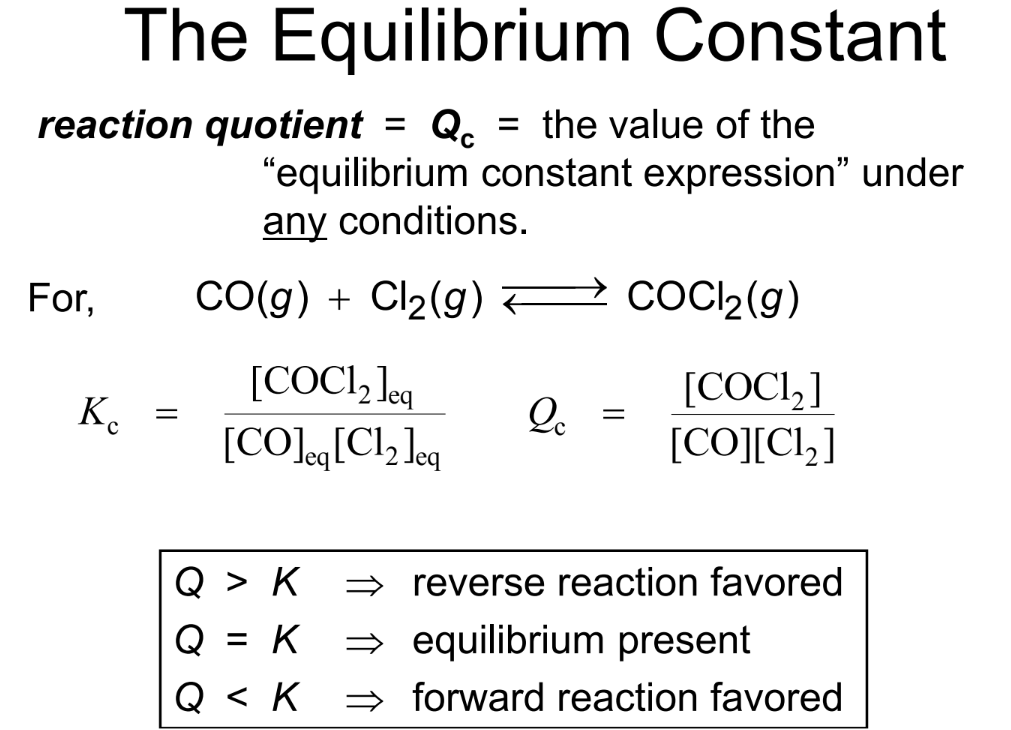
The Equilibrium Constant
At equilibrium, the rate of the forward reaction equals the rate of the reverse reaction.
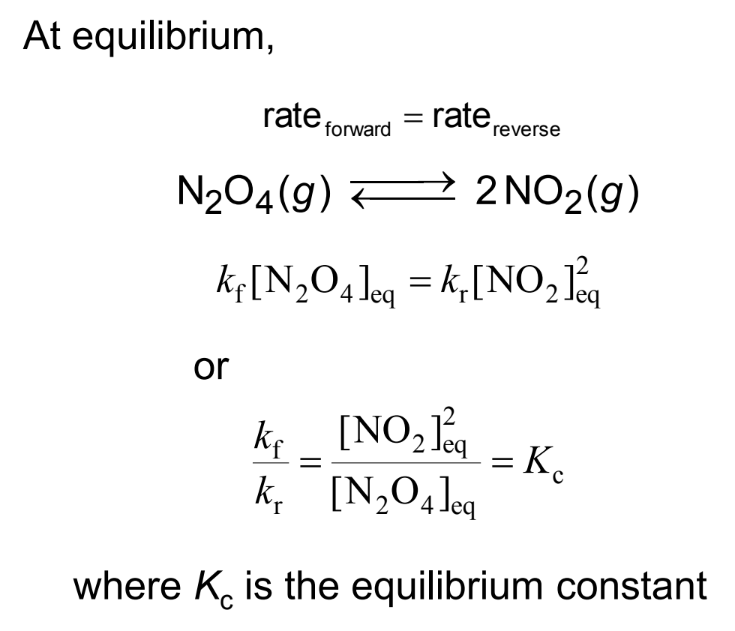
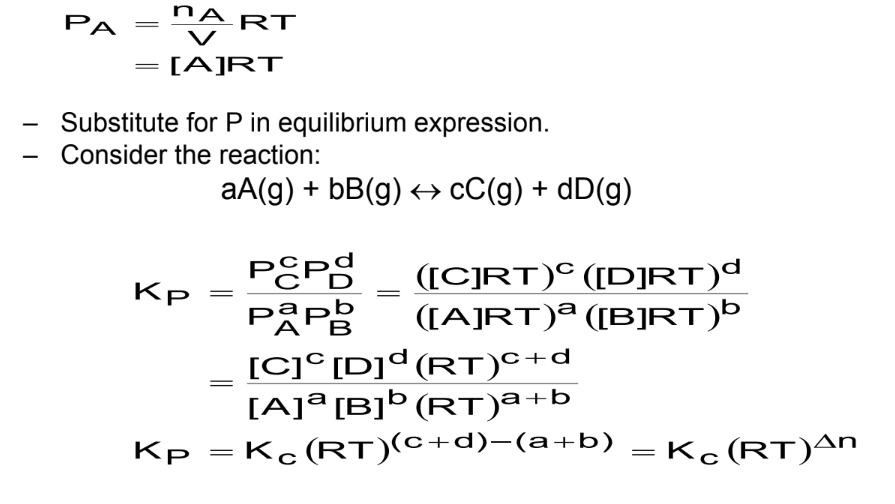
The relationship between the equilibrium constants Kc and Kp: For a chemical reaction can be derived using the ideal gas law.
Ideal Gas Law is PV=nRT
Equilibrium Expressions
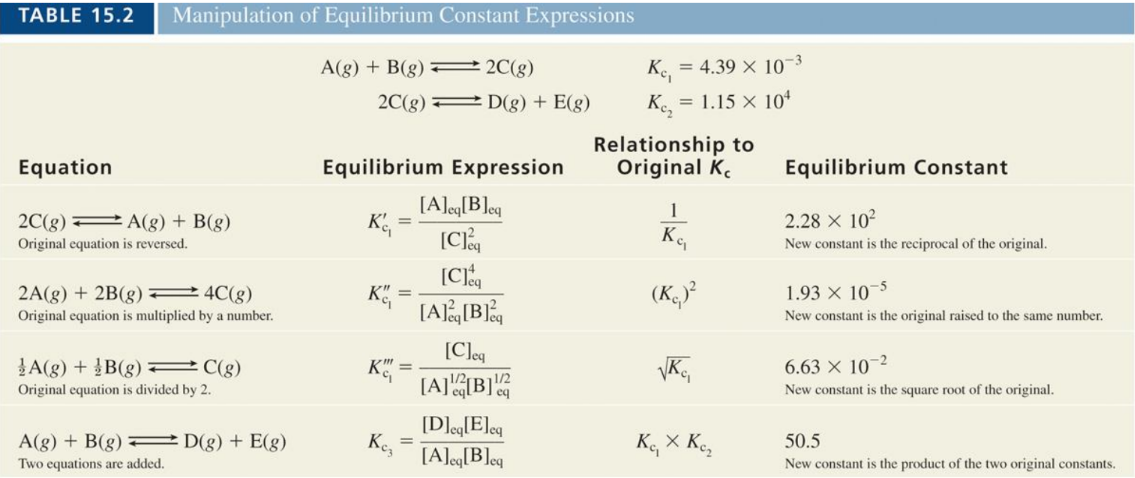
The Law of Mass Action
This law states that the rate of a chemical reaction is proportional to the product of the concentrations of the reactants, each raised to a power equal to the coefficient in the balanced equation.
Example: For the reaction N2O4(g)⇌2NO2(g)
Kc=[NO2]2[N2O4]
Homogeneous vs. Heterogeneous Equilibria
- Homogeneous Equilibria: All reactants and products are in the same phase (e.g., all gases).
- Heterogeneous Equilibria: Reactants and products are in different phases (e.g., solid and gas).
Example of Heterogeneous Equilibria:
CaCO3(s)⇌CaO(s)+CO2(g)
The equilibrium constant expression is:
K=[CO2]
Applications of the Equilibrium Constant
The equilibrium constant (KcK_cKc for concentration and KpK_pKp for pressure) is a fundamental concept in chemical equilibrium that has several important applications. Here are some of the key applications of the equilibrium constant:
1. Predicting the Direction of a Reaction
The equilibrium constant helps in predicting the direction in which a chemical reaction will proceed to reach equilibrium. By comparing the reaction quotient (QQQ) with the equilibrium constant (KKK), we can determine whether the reaction will shift towards the products or the reactants:
- If Q<K, the reaction will proceed in the forward direction (towards products) to reach equilibrium.
- If Q>K, the reaction will proceed in the reverse direction (towards reactants) to reach equilibrium.
- If Q=K, the system is already at equilibrium, and no net change will occur.
2. Calculating Equilibrium Concentrations
The equilibrium constant is used to calculate the concentrations of reactants and products at equilibrium. By setting up an equilibrium expression and using stoichiometric relationships, one can determine the unknown concentrations if the initial concentrations and K are known. This is commonly done using ICE tables (Initial, Change, Equilibrium) to organize the data and solve for the equilibrium concentrations.
3. Understanding Reaction Extent and Yield
The value of the equilibrium constant indicates the extent to which a reaction proceeds:
- A large K (much greater than 1) suggests that the reaction heavily favors the formation of products at equilibrium, meaning the products are predominant.
- A small K (much less than 1) indicates that the reaction favors the reactants, meaning very little product is formed, and the reactants are predominant at equilibrium.
Le Châtelier’s Principle: Restoring Balance
Le Châtelier’s Principle states that if a system at equilibrium is disturbed, the system will adjust itself to partially counteract the effect of the disturbance and restore a new equilibrium.
Factors Affecting an Equilibrium System
- Change in Concentration: Adding or removing a reactant or product shifts the equilibrium to oppose the change.
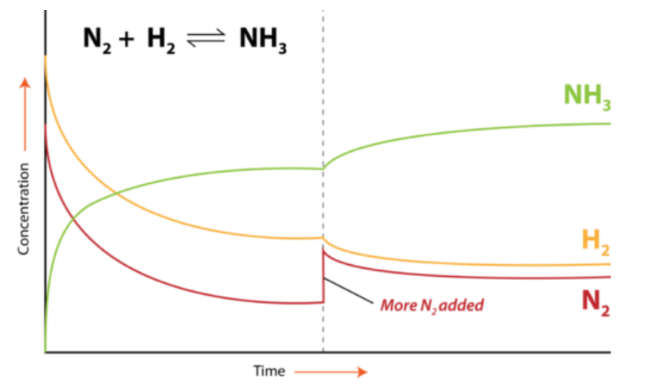
- Change in Temperature: Increasing the temperature favors the endothermic reaction; decreasing favors the exothermic reaction.
- Change in Pressure: Affects only reactions involving gases. Increasing pressure favors the side with fewer gas molecules, and decreasing favors the side with more gas molecules.
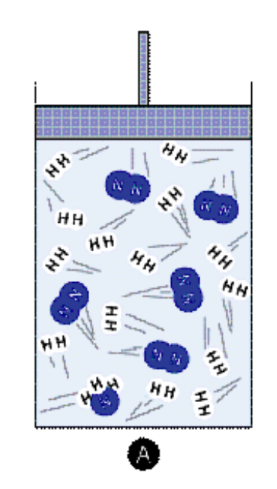
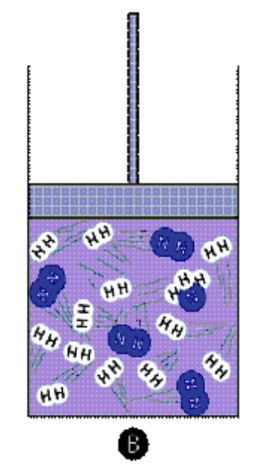
Use of Catalyst: A catalyst speeds up both the forward and reverse reactions equally, thus it does not change the equilibrium position but helps to reach equilibrium faster.
Conclusion
In summary, Chapter 6: Equilibrium from CBSE Class 11 Chemistry serves as an essential foundation for understanding both chemical and biological processes. By delving into the core concepts of equilibrium, students are equipped with the knowledge needed to grasp how dynamic systems maintain balance through various mechanisms. The principles discussed in Chapter 6: Equilibrium not only elucidate the laws governing chemical reactions, such as Le Chatelier’s principle and the equilibrium constant but also highlight the critical role of equilibrium in biological systems.
This chapter emphasizes the interconnectedness of equilibrium in everyday life, illustrating how organisms achieve homeostasis and respond to environmental changes. The resources provided, including interactive quizzes and real-life applications, ensure that students not only learn but also apply these concepts in practical contexts. Ultimately, a strong grasp of Chapter 6: Equilibrium will empower students to explore the intricate relationships within chemistry and biology, paving the way for further studies in these interconnected fields.
Practice questions on Chapter 6 - Equilibrium
Get your free Chapter 6 - Equilibrium practice quiz of 20+ questions & detailed solutions
Practice Now