Structure of Atom – Complete Guide For Class 11 Chemistry Chapter 2
Welcome to iPrep, your Learning Super App. Our learning resources for Chapter 2, “Structure of Atom,” in Class 11 Chemistry are meticulously designed to ensure students gain a comprehensive understanding of this essential topic. These resources include detailed notes on atomic theory, the arrangement of electrons in shells, and the quantum mechanical model of the atom. Students will also explore the concepts of atomic orbitals, electron configuration, and periodic trends. The materials provide visual aids and diagrams to illustrate the structure of atoms and include practice questions to solidify understanding. These resources are crafted to help students grasp the fundamental concepts necessary for studying the chemistry of biological systems.
Meaning of The Structure Of Atom
The concept of “Structure of Atom” in Class 11 Chemistry delves into the foundational principles of life by exploring the basic building blocks of matter. It examines the atomic theory, crucial for understanding how elements and compounds interact to form biological molecules. The chapter covers the arrangement of electrons in atoms and how this arrangement influences chemical bonding and molecular structure. This understanding is essential for comprehending how atoms combine to form complex biomolecules such as proteins, nucleic acids, and lipids. By linking the structure of atoms to chemical functions, students gain insight into the chemical basis of life processes.
Discovery of Subatomic Particles
The atom, once thought to be indivisible, was found to consist of smaller particles. The discovery of these subatomic particles electrons, protons, and neutrons revolutionized the understanding of the structure of the atom.
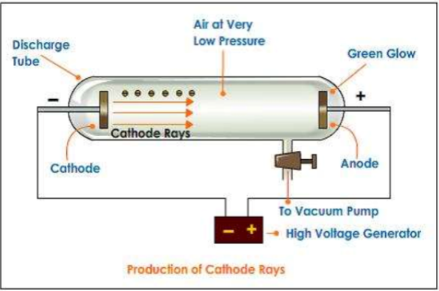
Properties of Cathode Rays
The chapter “Structure Of Atom” significantly covers the properties of cathode rays. These Cathode rays, discovered in the late 19th century, are streams of electrons emitted from the cathode in a vacuum tube. They exhibit several properties:
- Travel in straight lines.
- It causes fluorescence when it strikes certain materials.
- Are deflected by electric and magnetic fields, indicating they carry a negative charge.
- Possess energy and momentum.
Charge to Mass Ratio of Electron
J.J. Thomson, through his experiments with cathode rays, calculated the charge-to-mass ratio (e/m) of the electron. This was a significant step toward understanding the properties of the electron.
Charge on the Electron
The charge of an electron was determined by Robert Millikan through the oil drop experiment. The elementary charge was found to be approximately 1.602×10−191.602 \times 10^{-19}1.602×10−19 coulombs.
Discovery of Protons and Neutrons
- Protons: Positively charged particles found in the nucleus of the atom. Their discovery by Ernest Rutherford followed the identification of the electron.
- Neutrons: Discovered by James Chadwick, neutrons are neutral particles also located in the nucleus and contribute to the atom’s mass but not its charge.
Atomic Models
Various atomic models have been proposed to explain the structure of atoms:
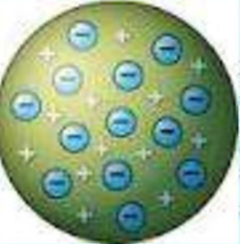
Thomson model, also known as the “plum pudding model” of the atom, can be described with the following key points:
- Uniform Positive Charge: J.J. Thomson proposed that an atom consists of a uniformly distributed positive charge (like a “pudding”) in which electrons (the “plums”) are embedded. This positive charge was thought to balance out the negative charge of the electrons, making the atom electrically neutral.
- Electron Distribution: According to this model, electrons were scattered throughout the positively charged “pudding” like raisins in a plum pudding. This suggested that electrons were stationary within the atom and did not have any specific arrangement or orbit.
Rutherford’s Nuclear Model of Atom
Rutherford’s gold foil experiment led to the nuclear model of the atom, proposing that an atom consists of a dense, positively charged nucleus surrounded by electrons.
Explanation of Rutherford’s Nuclear Model of Atom
In Rutherford’s model:
- The nucleus contains protons and neutrons, making it the atom’s center of mass.
- Electrons orbit the nucleus, similar to planets orbiting the sun.
- Most of the atom’s volume is in space.
Main Points of Rutherford’s Nuclear Model of Atom
- Nucleus: A dense core containing protons and neutrons.
- Electrons: Negatively charged particles orbiting the nucleus.
- Empty Space: Most of the atom’s volume is empty.
Atomic Number and Mass Number
- Atomic Number (Z): The number of protons in the nucleus of an atom, defining the element.
- Mass Number (A): The total number of protons and neutrons in an atom’s nucleus.
Isobars and Isotopes
- Isobars: Atoms with the same mass number but different atomic numbers (e.g., 1840Ar^{40}_{18}\text{Ar}1840Ar and 2040Ca^{40}_{20}\text{Ca}2040Ca).
- Isotopes: Atoms with the same atomic number but different mass numbers (e.g., 612C^{12}_{6}\text{C}612C, 613C^{13}_{6}\text{C}613C, and 614C^{14}_{6}\text{C}614C).
Drawbacks of Rutherford’s Model
While revolutionary, Rutherford’s model had drawbacks:
- It couldn’t explain the stability of atoms, as electrons spiraling into the nucleus should theoretically collapse.
- It didn’t account for the discrete line spectra of elements.
Developments Leading to The Bohr’s Model of Atom
The limitations of Rutherford’s model paved the way for new theories, incorporating quantum concepts and leading to Niels Bohr’s model.
Particle Nature of Electromagnetic Radiation: Planck’s Quantum Theory
Let’s now discuss Plank’s quantum theory covered in the chapter Structure of Atom. Max Planck proposed that electromagnetic energy is quantized and can be emitted or absorbed in discrete quantities called quanta or photons.
Planck’s Quantum Theory II
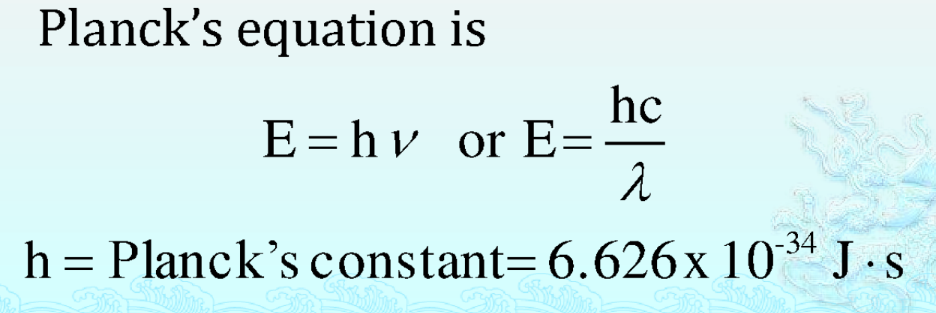
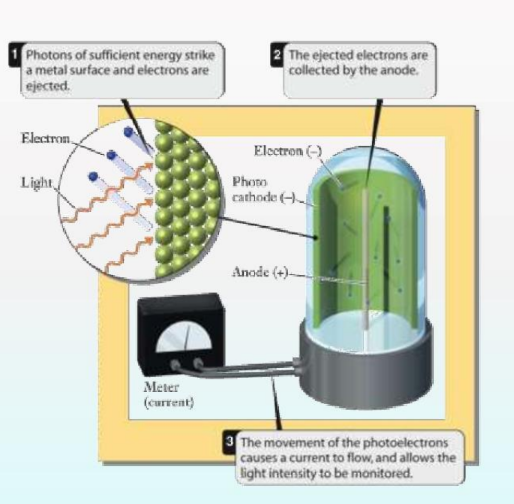
Photoelectric Effect
Einstein’s explanation of the photoelectric effect showed that light behaves as particles (photons). When light of sufficient energy strikes a metal surface, it emits electrons. This phenomenon confirmed the particle nature of electromagnetic radiation.
Dual Behaviour of Electromagnetic Radiation
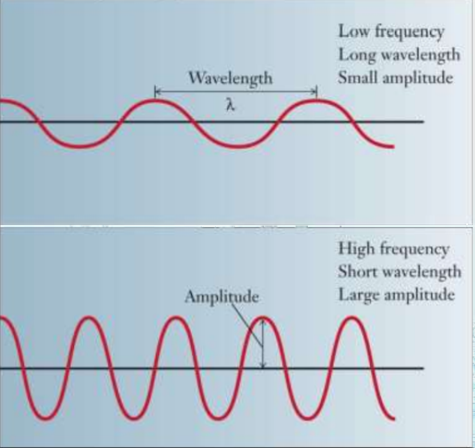
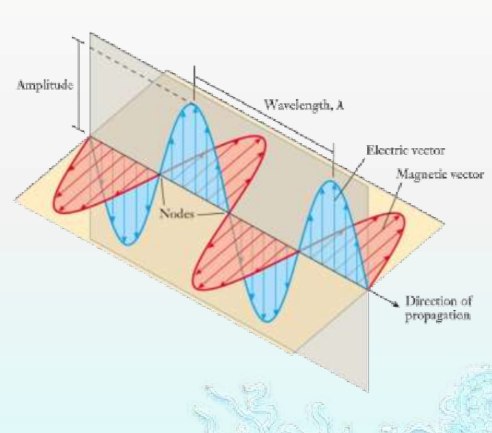
Electromagnetic radiation, as stated in the chapter Structure of Atom, exhibits both wave-like and particle-like properties, a concept known as wave-particle duality.
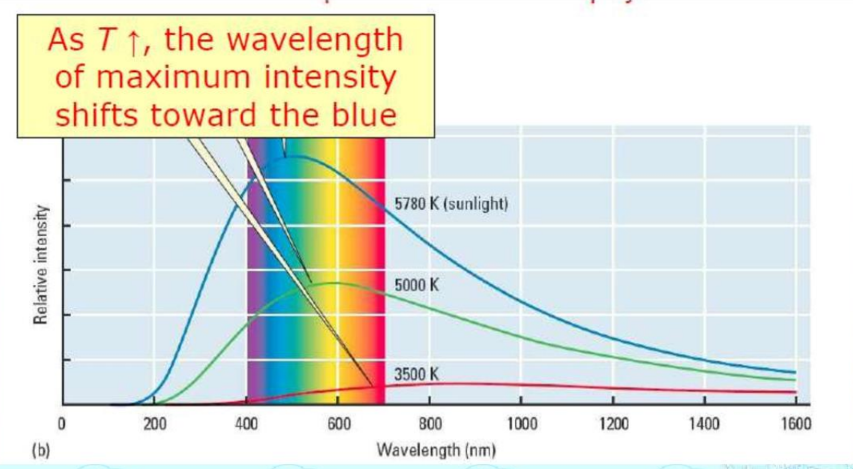
Evidence for The Quantized Electronic Energy Levels: Atomic Spectra
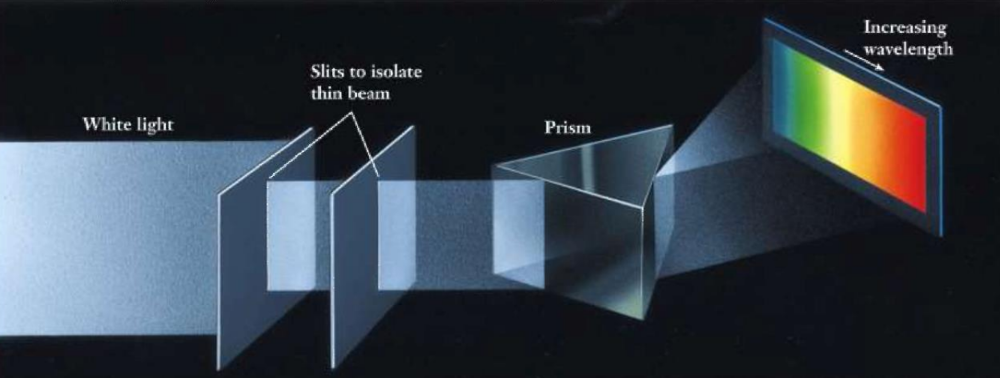
As covered in the chapter structure of atoms, Atoms emit or absorb light at specific wavelengths, forming an atomic spectrum. This spectrum provides evidence for quantized energy levels within an atom.
Emission and Absorption Spectra
- Emission Spectrum: Produced when an electron drops from a higher energy level to a lower one, emitting energy as light.
- Absorption Spectrum: Formed when electrons absorb energy and move to higher energy levels, leaving dark lines in the spectrum.
Line Spectrum of Hydrogen
The hydrogen atom’s line spectrum, with distinct lines in the visible region, led to the understanding that electrons occupy specific energy levels.
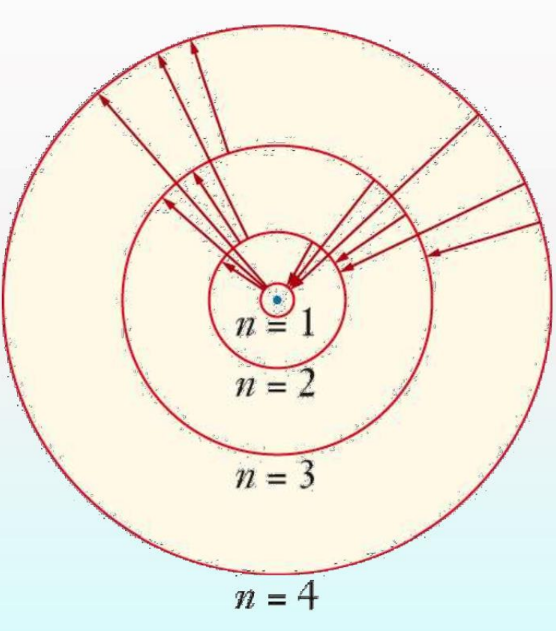
Bohr’s Model for Hydrogen Atom
Within the chapter structure of atom, Bohr’s model addressed the shortcomings of Rutherford’s model by introducing quantized orbits for electrons.
Bohr’s Model for Hydrogen Atom
Key postulates of Bohr’s model:
- Electrons revolve around the nucleus in certain fixed orbits without emitting radiation.
- Energy is emitted or absorbed only when an electron transitions between these orbits.
- The angular momentum of an electron in these orbits is quantized and given by mvr=nh2πmvr = n\frac{h}{2\pi}mvr=n2πh, where nnn is the principal quantum number.
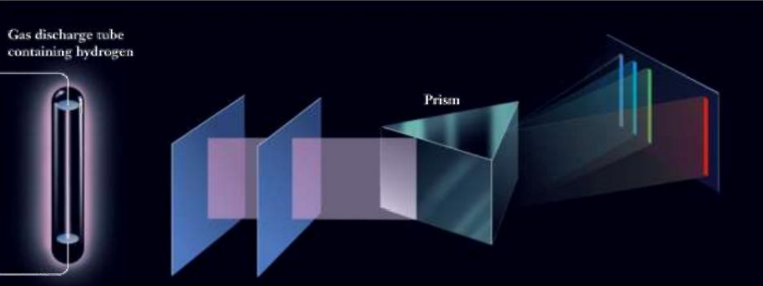
Explanation of Line Spectrum of Hydrogen
Bohr’s model explained the hydrogen atom’s line spectrum by associating spectral lines with electron transitions between quantized orbits.
Limitations of Bohr’s Model
Despite its success, Bohr’s model had limitations:
- It couldn’t explain the spectra of atoms with more than one electron.
- It didn’t consider electron-electron interactions and relativistic effects.
Reasons for The Failure of The Bohr Model
As mentioned in the chapter Structure of Atom, the Bohr model failed for multi-electron systems because:
- It did not incorporate the wave nature of electrons.
- It could not explain the fine structure and splitting of spectral lines.
Towards Quantum Mechanical Model of The Atom
The quantum mechanical model developed from the need to address the limitations of Bohr’s model and incorporate the wave-particle duality of electrons.
Heisenberg’s Uncertainty Principle
Heisenberg’s uncertainty principle states that it is impossible to simultaneously determine the exact position and momentum of an electron.
Significance of the Uncertainty Principle
This principle implies that the concept of definite paths or orbits for electrons, as in the Bohr model, is incorrect.
Example
If the uncertainty in position (Δx\Delta xΔx) is reduced, the uncertainty in momentum (Δp\Delta pΔp) increases, and vice versa.
Quantum Mechanical Model of Atom
The quantum mechanical model describes electrons in terms of probability distributions, rather than fixed orbits, using wave functions.
Significance of Ψ
Ψ (Psi) represents the wave function of an electron, and ∣Ψ∣2|Ψ|^2∣Ψ∣2 gives the probability density of finding an electron in a particular region around the nucleus.
Orbitals and Quantum Numbers
An orbital is a region in space where the probability of finding an electron is highest. Quantum numbers describe the properties of these orbitals.
The Principal Quantum Number (n)
Indicates the main energy level or shell, with values of n=1,2,3,…n = 1, 2, 3, \ldotsn=1,2,3,….
Azimuthal Quantum Number (l)
Defines the shape of the orbital, with values ranging from 000 to n−1n-1n−1.
Magnetic Orbital Quantum Number (ml)
Describes the orientation of the orbital in space, with values ranging from −l-l−l to +l+l+l.
Spin Quantum Number (ms)
Specifies the spin orientation of the electron, with values of ±12\pm \frac{1}{2}±21.
Shapes of Atomic Orbitals
Atomic orbitals have different shapes based on the azimuthal quantum number:
Shapes of Atomic Orbitals I
- s-orbital: Spherical in shape.
- P-orbital: Dumbbell-shaped.
Shapes of Atomic Orbitals II
- d-orbital: Four-lobed or cloverleaf shape.
- F-orbital: Complex shapes with multiple lobes.
Charge Cloud Picture of Orbitals
The charge cloud model represents the probability distribution of an electron’s position, emphasizing areas of higher probability as denser clouds.
Energies of Orbitals
The energy of an orbital depends on its principal quantum number and azimuthal quantum number.
Energies of Orbitals I
In a single-electron atom, the energy depends only on the principal quantum number.
Energies of Orbitals II
In multi-electron atoms, electron-electron interactions cause energy levels to split further.
Filling of Orbitals in Atom
Electrons fill orbitals in a way that minimizes the atom’s energy, following the Aufbau principle, Pauli exclusion principle, and Hund’s rule.
Hund’s Rule of Maximum Multiplicity
Electrons occupy degenerate orbitals singly before pairing up to maximize the number of unpaired electrons.
Electronic Configuration of Atoms
The chapter structure of atoms also covers the Electronic Configuration of Atoms. The electronic configuration of an atom describes the distribution of electrons among orbitals.
Electronic Configuration of Atoms I
Configuration follows the order of increasing energy levels: 1s,2s,2p,3s,3p,4s,1s, 2s, 2p, 3s, 3p, 4s,1s,2s,2p,3s,3p,4s, etc.
Electronic Configuration of Atoms II
- Example: The electronic configuration of carbon (Z=6Z = 6Z=6) is 1s22s22p21s^2 2s^2 2p^21s22s22p2.
Stability of Completely Filled and Half-Filled Subshells
According to the structure of atoms, Atoms with filled or half-filled subshells exhibit extra stability due to symmetrical distribution and exchange of energy.
Conclusion
This comprehensive guide on “Structure of Atom” provides an in-depth exploration of the fundamental aspects of CBSE Chemistry as outlined in Class 11. It covers the core concepts of atomic theory, including the historical development of atomic models and the current quantum mechanical model. The guide delves into the arrangement of electrons in different orbitals and shells, and how this arrangement affects chemical properties and bonding. It also explores the periodic trends in the structure of atoms and their relevance to the behavior of elements in biological systems. By integrating these concepts with biological applications, the guide helps students understand the role of the structure of atoms in the chemistry of life.
Practice questions on Chapter 2 - Structure Of Atom
Get your free Chapter 2 - Structure Of Atom practice quiz of 20+ questions & detailed solutions
Practice Now








