A Journey through States of Water – Complete Guide For Class 6 Science Chapter 8
Welcome to iPrep, your Learning Super App. Our learning resources for the chapter, A Journey through States of Water in Science for Class 6th are designed to ensure that you grasp this concept with clarity and perfection. Whether you’re studying for an upcoming exam or strengthening your concepts, our engaging animated videos, practice questions and notes offer you the best of integrated learning with interesting explanations and examples.
Introduction to the States of Water
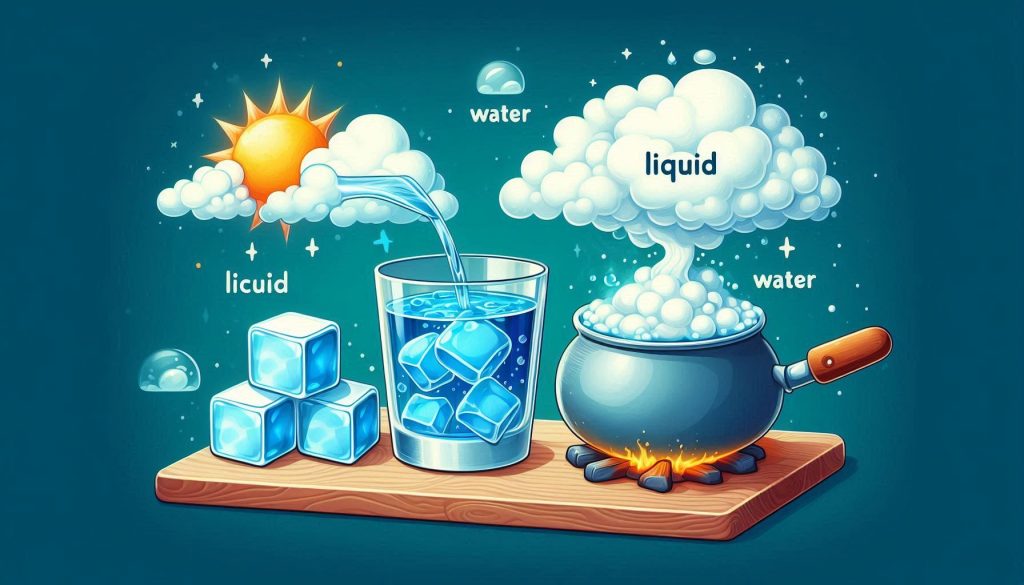
Water is an essential part of our life. There exist various states of water including —solid, liquid, and gas. Have you ever wondered why ice feels hard, while water flows freely? Or how water seemingly vanishes from puddles after rain? In this chapter, we will explore the fascinating journey of water as it transitions between its different states, from ice to liquid to water vapor, and back again.
Investigating Water’s Disappearing Act
Have you ever noticed how water in puddles disappears after rain? Aavi and Thirav observed this during their school day and wondered where the water went. When water on utensils also dries after washing, could it be the same process? Through an activity given in the chapter – states of water, we learn that water doesn’t seep through steel plates; it evaporates into the air.
Activity: Observing States Of Water
- Steps:
- Place an ice cube in a cup and let it sit.
- Observe how the ice melts into water. This simple observation shows how ice (solid) changes into water (liquid), proving they are the same substance in different states.
Understanding Evaporation
As mentioned in the chapter States of Water, Evaporation is the process through which water changes from a liquid state into vapor. For example, when water is sprinkled on a hot pan, it quickly turns into steam, which is water vapor. This process happens continuously in our environment, such as when wet clothes dry or when water puddles disappear.
Activity: Investigating Evaporation
- Materials: A tablespoon of water on a steel plate.
- Steps:
- Observe how the water disappears without seeping through the plate.
- This demonstrates that water turns into water vapor, another state of water.
Water converts into water vapor, a gaseous state of water, through evaporation. For example, when sprinkling water on a hot pan while making dosa, the water turns into steam, which is water vapour, with some converting back into droplets. Evaporation occurs continuously, even at room temperature, and can be seen in daily life, like drying clothes, a mopped floor, or sweat. The disappearance of water from puddles is due to evaporation or a combination of evaporation and seeping into the ground. Similarly, hand sanitizer evaporates as it is rubbed on the hands.
The Mystery of Condensation
Condensation according to the chapter states of water, is the process where water vapor turns back into liquid water. Aavi and Thirav noticed water droplets forming on the outer surface of a glass filled with ice water. But where did these droplets come from? This is an example of condensation, where the water vapor in the air comes into contact with the cold surface and forms droplets.
Activity: Observing Condensation
- Steps:
- Take a glass of cold water with ice cubes.
- Observe how water droplets form on the outer surface.
Dew drops are more visible in the morning because water vapour in the air condenses into droplets when it comes into contact with cooler surfaces. Similarly, when boiling water in a covered utensil, water vapour condenses on the inner side of the steel plate, forming droplets. This process, known as condensation, occurs when water vapour turns into liquid as mentioned in the chapter states of water.
In Activity, a glass tumbler with cold water and ice cubes shows condensation on its outer surface as water vapour from the air contacts the cold surface. The weight of the tumbler increases, suggesting that condensation is responsible for the water droplets. A modified version of the experiment, marking the water level, shows the water level inside the tumbler remains constant, confirming that no water seeps from the tumbler and the collected water outside is due to condensation.
This shows that water vapor from the air condenses into liquid when it touches a cold surface.
The Three States of Water
There are three major states of water: solid, liquid, and gas. Ice (solid) melts into water (liquid), and water can evaporate into water vapor (gas). Each state has different properties:
- Solid (Ice): Retains its shape and does not flow.
- Liquid (Water): Takes the shape of its container and flows freely.
- Gas (Water Vapor): Spreads out to fill the available space.
Activity: Identifying States of Water
- Steps:
- Transfer ice cubes from one container to another and observe how they retain their shape.
- Pour water into different containers and observe how it takes the shape of the container.
- Pour water on a surface and see how it spreads.
Water exists in three states: solid, liquid, and gas, each with distinct properties. In its solid state (ice), water retains its shape and does not flow. In the liquid state, water takes the shape of its container, flows, and spreads, but its volume remains constant. Water vapour, in the gaseous state, spreads to fill the entire space and is invisible, though present in the air around us.
Other substances, like wax, oil, and ghee, also exhibit these states. Solid examples include stones, wood, and glass, while liquids like milk and oil can be identified. Gases, such as oxygen and carbon dioxide, spread through the air, much like the smell of food from the kitchen reaching us from a distance.
Changing the States of Water
Heating or cooling can change the states of water. Ice melts into water when heated, and water boils into vapor when heated further. On the other hand, cooling water turns it into ice.
Activity: Completing the Diagram
Fill in the blanks in the following diagram to show how water transitions between solid, liquid, and gas:
Process State Transition Melting Ice → Water Freezing Water → Ice Evaporation Water → Water Vapor Condensation Water Vapor → Water
Factors Affecting Evaporation
According to the science behind the states of water, Evaporation is influenced by several conditions. It is observed that water evaporates faster when spread out over a larger area, as in a plate compared to a bottle cap. This shows that the exposed surface area affects the evaporation rate.
If we place two bottle caps with equal water in sunlight and shade it will be observed that water evaporates faster in sunlight. Similar results are observed on windy days, where clothes dry faster due to increased air movement, while on rainy days, high humidity slows down evaporation.
To dry clothes faster on a rainy day, one could use a fan or place the clothes in a less humid environment to encourage faster evaporation.
Cooling Effect of Evaporation
Evaporation causes a cooling effect. For example, water in an earthen pot stays cooler because water seeps through the surface and evaporates, cooling the pot. Similarly, rubbing sanitizer on your hands feels cool because it evaporates quickly.
Activity: Making a Pot-in-Pot Cooler
- Steps:
- Take two earthen pots, one smaller than the other.
- Place the smaller pot inside the larger one, filling the gap with sand.
- Add water to the sand and cover the pots with a wet cloth.
This simple cooler keeps fruits and vegetables fresh by using the cooling effect of evaporation.
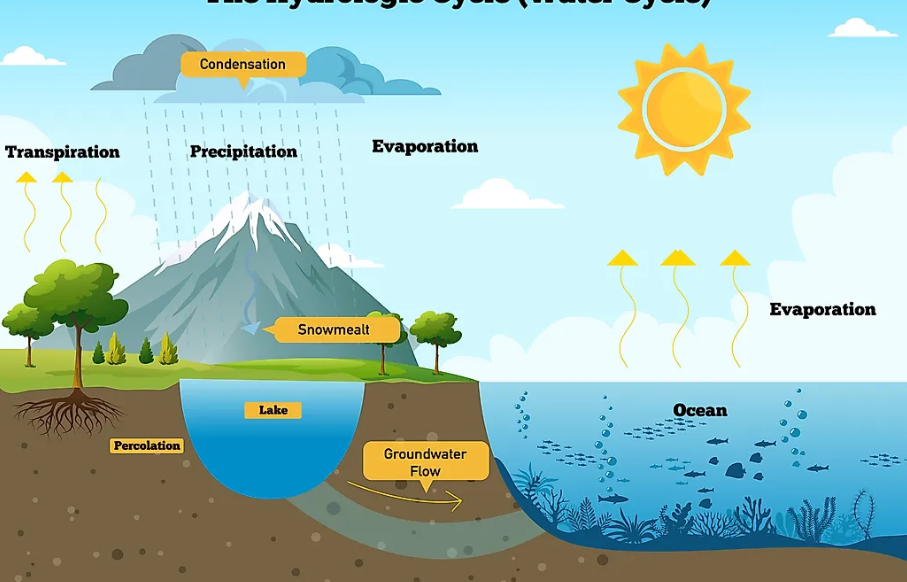
How Do Clouds Give Us Rain?
As stated in the chapter- states of water, Clouds form when water vapor in the air condenses around dust particles. As the water droplets combine, they grow larger and eventually fall as rain. This process is part of the water cycle, which circulates water between the Earth and the atmosphere.
Activity: Understanding Cloud Formation
- Steps:
- Take a plastic bottle filled with a small amount of water and add a burnt piece of newspaper.
- Squeeze and release the bottle continuously to see how clouds form inside.
The Water Cycle
The water cycle refers to the continuous movement of water as it evaporates from oceans and Earth’s surface into the atmosphere and returns as rain, hail, or snow, eventually flowing back to the oceans. Only a small amount of Earth’s water is usable by plants, animals, and humans, with most of it in the oceans. As the population increases, so does water demand, leading to shortages in many regions. Therefore, it is essential to use water wisely, avoid wastage, and prevent water pollution.
Summary of Key Concepts
- Evaporation: The process of water turning into vapor.
- Condensation: The conversion of water vapor into liquid.
- The Water Cycle: The circulation of water between the Earth and the atmosphere.
- Cooling Effect: Evaporation causes cooling, such as in an earthen pot.
Let’s Conclude
In conclusion, CBSE Class 6th Science Chapter 8 – A Journey through States of Water provides a captivating exploration of the different states of water—solid, liquid, and gas. Through engaging activities and observations, students learn about the processes of evaporation and condensation, understanding how water transitions between these states in everyday life. The chapter emphasizes the significance of the water cycle and its vital role in our ecosystem, reminding us to conserve this precious resource.
By delving into A Journey through States of Water, students not only grasp the scientific concepts but also develop a deeper appreciation for the world around them. With the resources available on the iPrep Learning Super App, learners can strengthen their understanding and excel in their studies. So, let’s embark on this exciting journey through the states of water and appreciate the wonders of nature!
Practice questions on Chapter 8 - States Of Water
Get your free Chapter 8 - States Of Water practice quiz of 20+ questions & detailed solutions
Practice Now