Matter in Our Surroundings – Complete Guide For Class 9 Science Chapter 1
Welcome to iPrep, your Learning Super App. Our learning resources for the chapter, Matter in Our Surroundings in Science Class 9th Chapter 1 are designed to ensure that you grasp this concept with clarity and perfection. Whether you’re studying for an upcoming exam or strengthening your concepts, our engaging animated videos, practice questions and notes offer you the best of integrated learning with interesting explanations and examples.
The concept of “Matter in Our Surroundings” in Class 9 Science introduces students to the fundamental building blocks of chemistry. It provides a comprehensive overview of the physical and chemical nature of matter, its various states, and the processes that cause it to change from one state to another. By understanding these basic principles, students can develop a strong foundation for more advanced topics in chemistry and gain insights into the practical applications of these concepts in everyday life.
What is Matter?
Everything around us is made of matter like the air we breathe, the water we drink, the food we eat, the stars, plants, animals, and even we ourselves are composed of matter. Early Indian and Greek philosophers classified matter into five basic elements of Panch Tatva: air, water, earth, sky, and fire. Today, scientists classify matter based on physical and chemical properties. Simply put, anything that has mass and occupies space is matter.
Physical Nature of Matter
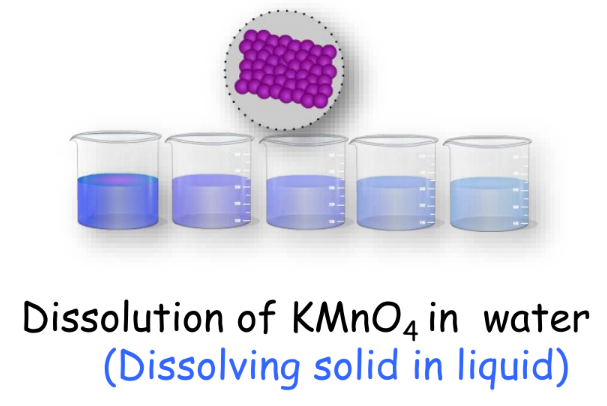
Every matter in our surroundings is made up of particles. Historically, scientists debated whether matter in our surroundings was continuous or particulate. Experiments, such as dissolving potassium permanganate (KMnO4) in water, demonstrate the particulate nature of matter. Even a small amount of Dettol can diffuse into water, illustrating the small, interspaced, and continuously moving particles of matter.
Characteristics of Particles of Matter
- Very Small: Particles are so small they cannot be seen with the naked eye.
- Space Between Particles: Particles have spaces between them.
- Continuous Movement: Particles are in continuous motion.
- Attraction: Particles attract each other.
- Kinetic Energy: Particles possess kinetic energy.
States of Matter
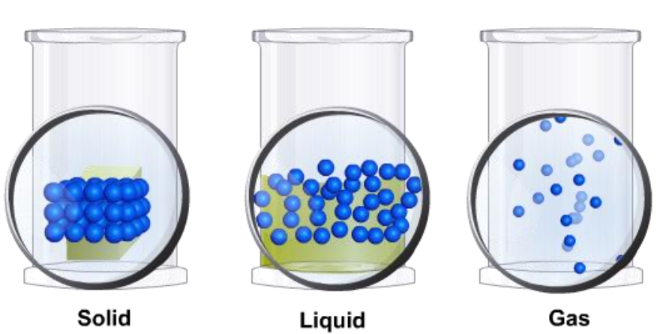
Matter in our surroundings exists in three primary states based on the physical characteristics of its particles: solid, liquid, and gas.
Solid State
In the solid state, matter in our surroundings has particles that have
- Fixed Shape: Particles are closely packed, resulting in a fixed shape.
- Fixed Volume and Boundaries: Strong attractive forces between particles give solids a fixed volume and rigid structure.
- Incompressible: Small spaces between particles make solids incompressible.
- No Diffusion: Solids do not diffuse into other solids.
Examples:
- Sponge: Compressible due to air trapped in pores.
- Sugar Crystals: Maintain shape despite the container’s shape.
Liquid State
The liquid state is a key state of matter in our surroundings where there is
- No Fixed Shape: Particles are not closely packed, allowing liquids to take the shape of their container.
- Fixed Volume: Attractive forces keep particles together.
- Fluidity: Liquids can flow and are more compressible than solids.
- Diffusion: Liquids can diffuse into each other.
Gaseous State
The gaseous state is one of the fundamental states of matter, characterized by –
- No Definite Shape or Volume: Gases take the shape and volume of their container.
- Highly Compressible: Large spaces between particles.
- Fluidity: Gases can flow easily.
- Low Density: Particles are widely spaced.
- High Diffusion Rate: Gases diffuse faster than liquids.
Diffusion
Diffusion is a key process that illustrates the particulate nature of matter in our surroundings. It is the movement of particles from high to low concentration. It occurs in solids, liquids, and gases and is affected by the nature of the substance and temperature. For example, red ink diffuses faster in water than honey, and salt dissolves quickly in hot water.

Temperature and Its Measurement
Temperature plays a crucial role in determining the state and behavior of matter in our surroundings. Temperature measures the degree of hotness of an object. It can be measured in Kelvin (SI unit), Celsius, or Fahrenheit. The relation between Celsius and Kelvin is: K = 273 + °C.
Change of State
Matter in or surroundings can change states by altering temperature or pressure. The process of changing states is called the interconversion of matter. For example-
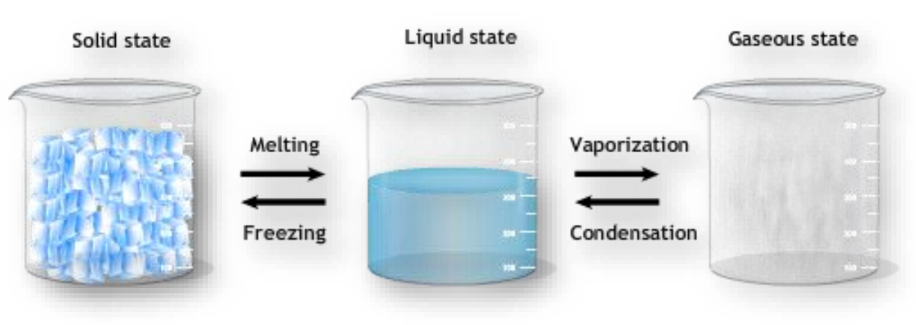
Solid to Liquid (Melting/Fusion)
Melting is the process where solid matter in our surroundings change into liquids by absorbing heat. The melting point is the constant temperature at which this occurs.
Liquid to Solid (Freezing/Solidification)
Freezing is the reverse of melting, where liquids change into solids by releasing heat. The freezing point is the constant temperature at which this occurs.
Liquid to Gas (Boiling/Vaporization)
Boiling is the process where liquids change into gases by absorbing heat. The boiling point measures the strength of attractive forces between particles.
Gas to Liquid (Condensation/Liquefaction)
Condensation is the process where gases change into liquids by releasing heat. This is the reverse of boiling.
Sublimation
Sublimation is a unique process where matter transitions directly from a solid to a gas without passing through the liquid state, as seen in substances like naphthalene and dry ice.
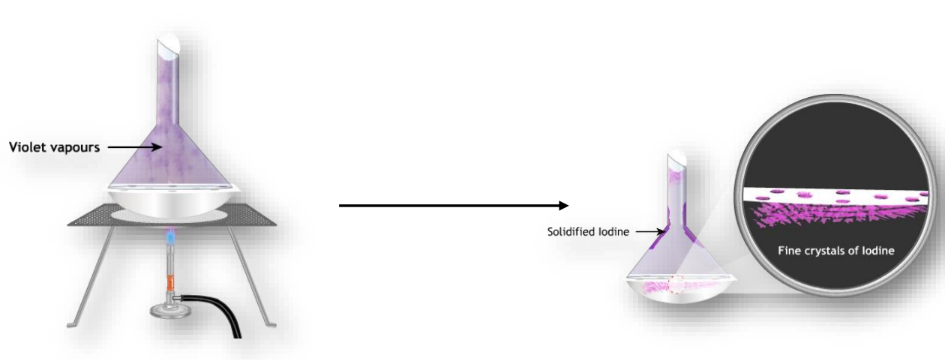
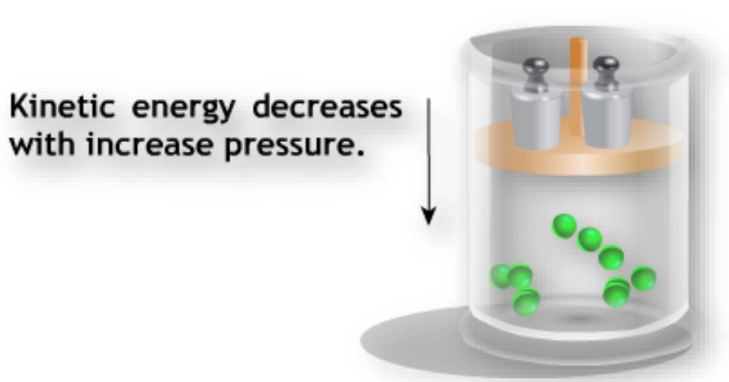
Effect of Pressure on States of Matter
Increasing pressure can change gases into liquids and solids. For example, applying high pressure to gaseous carbon dioxide forms solid dry ice.
Evaporation
Evaporation is the process where matter in its liquid state transforms into a gas at temperatures below its boiling point. Factors affecting evaporation include humidity, temperature, wind speed, and surface area.
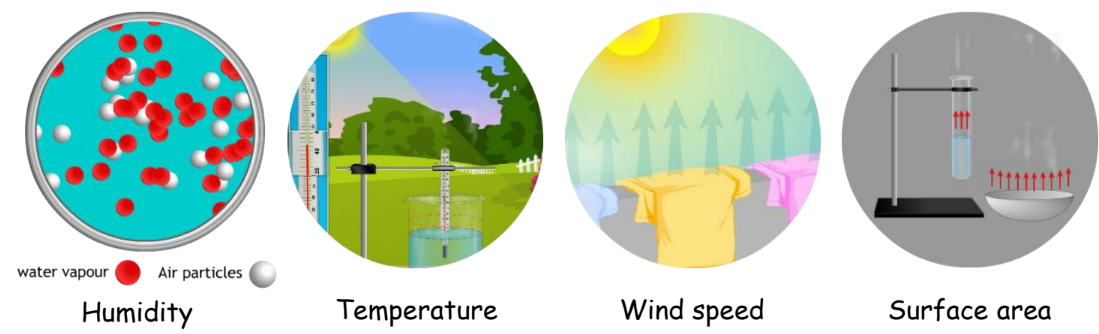
Practical Examples of Evaporation
- Drying Clothes: Clothes dry faster in the summer than in the winter.
- Cooling Effects: Sprinkling water on the ground cools it due to evaporation.
This comprehensive guide offers an in-depth exploration of the characteristics and behaviors of matter in different states, such as solids, liquids, and gases. It delves into the nature of particles, diffusion, and the effects of temperature and pressure on the state of matter. Understanding these properties helps us comprehend the world around us, from the food we eat to the air we breathe. Moreover, it allows us to harness this knowledge in various scientific and industrial applications, from creating new materials to improving energy efficiency and environmental sustainability.
In conclusion, the chapter Matter in Our Surroundings from Class 9 Science provides students with a fundamental understanding of the properties and behaviors of matter in its various states—solid, liquid, and gas. Through this chapter, students explore the physical and chemical nature of matter and the processes that cause it to change from one form to another, laying the groundwork for future studies in chemistry.
At iPrep, our engaging learning resources for Matter in Our Surroundings in Science Class 9th Chapter 1 are designed to make these concepts easy to grasp. Whether you’re preparing for exams or building a solid conceptual foundation, our interactive videos, notes, and practice exercises are here to guide you every step of the way.
Mastering the content of Matter in Our Surroundings will not only help in academic success but also in understanding the world around us, as matter is everywhere. So, dive into the fascinating world of science with iPrep, and make your learning experience enjoyable and effective with Chapter 1 – Matter in Our Surroundings.
Practice questions on Chapter 1 - Matter in Our Surroundings
Get your free Chapter 1 - Matter in Our Surroundings practice quiz of 20+ questions & detailed solutions
Practice Now








