Structure of the Atom – Complete Guide For Class 9 Science Chapter 4
Welcome to iPrep, your Learning Super App. Our learning resources for the chapter, Structure of the Atom in Science Class 9th Chapter 4 are designed to ensure that you grasp this concept with clarity and perfection. Whether you’re studying for an upcoming exam or strengthening your concepts, our engaging animated videos, practice questions and notes offer you the best of integrated learning with interesting explanations and examples.
The concept of “Structure of the Atom” in Class 9 Science introduces students to the fundamental building blocks of matter, providing a detailed understanding of how atoms are composed and how they function. It covers the historical development of atomic theory, the discovery of subatomic particles, and the various models proposed by scientists to explain atomic structure. This topic also explores concepts such as atomic number, mass number, isotopes, and electronic configuration, helping students grasp the intricate details of atomic composition. By studying the structure of the atom, students gain a foundational understanding that is essential for further studies in chemistry and related scientific disciplines.
Introduction to the Structure Of The Atom
Atoms are the fundamental building blocks of matter. Understanding the structure of the atom is crucial for comprehending the nature of substances and their interactions. This topic provides an in-depth exploration of atomic models, the discovery of subatomic particles, and the arrangement of electrons within atoms.
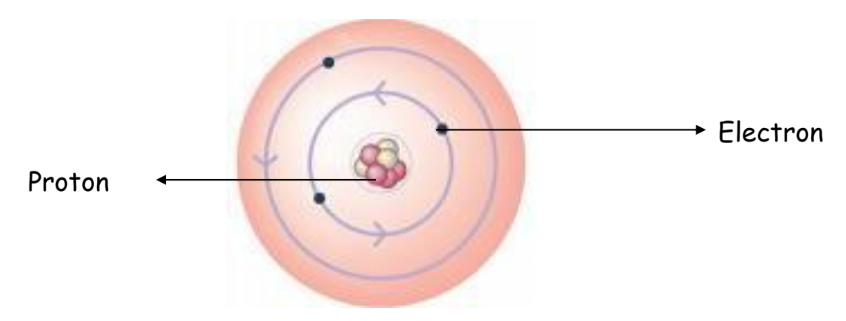
Early Models of the Atom
- Dalton’s Atomic Theory: John Dalton proposed that atoms are indivisible particles that make up all matter. He suggested that each element consists of identical atoms, and compounds form by combining atoms in fixed ratios.
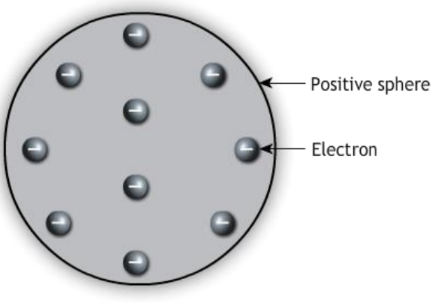
- Thomson’s Model: J.J. Thomson discovered the electron and proposed the “plum pudding” model, where electrons were embedded in a positively charged “soup.”
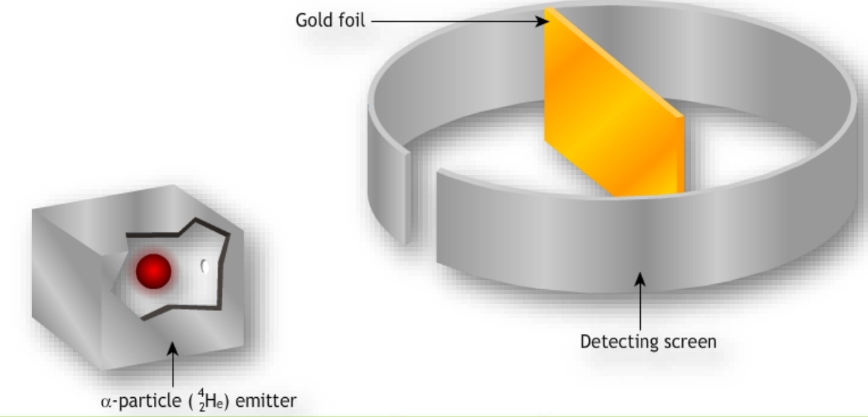
- Rutherford’s Model: Ernest Rutherford conducted the gold foil experiment, revealing that atoms have a small, dense nucleus surrounded by electrons. This led to the nuclear model of the atom.
Discovery of Subatomic Particles
- Electrons: Discovered by J.J. Thomson, electrons are negatively charged particles that orbit the nucleus.
- Protons: Discovered by Ernest Rutherford, protons are positively charged particles located within the nucleus.
- Neutrons: Discovered by James Chadwick, neutrons are neutral particles that also reside in the nucleus.
Bohr’s Model of the Atom
Niels Bohr refined Rutherford’s model by introducing quantized electron orbits. He proposed that electrons travel in specific orbits around the nucleus and can jump between these orbits by absorbing or emitting energy.
Electron Configuration
- Energy Levels: Electrons occupy discrete energy levels or shells around the nucleus, labeled K, L, M, and so on.
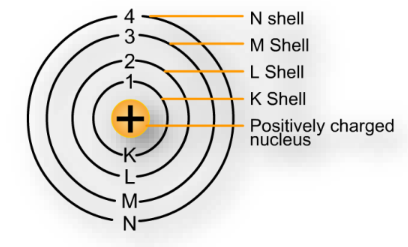
- Subshells and Orbitals: Each energy level contains subshells (s, p, d, f), which further contain orbitals. Electrons fill these orbitals following specific rules.
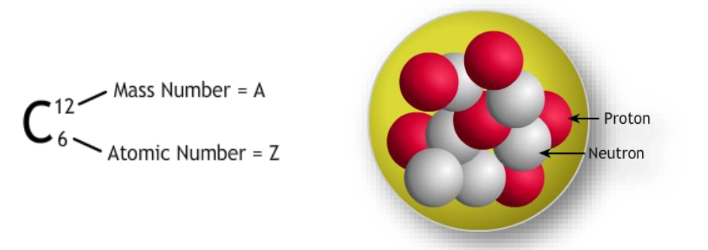
Atomic Number and Mass Number
- Atomic Number (Z): The number of protons in the nucleus, which determines the element’s identity.
- Mass Number (A): The total number of protons and neutrons in the nucleus.
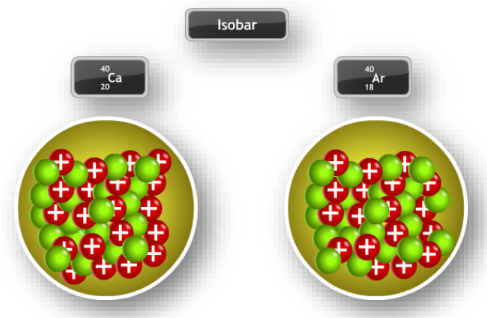
Isotopes
Isotopes are atoms of the same element with different mass numbers, due to varying numbers of neutrons. For example, Carbon-12 and Carbon-14 are isotopes of carbon.
Applications of Atomic Structure
Understanding atomic structure is fundamental in fields such as chemistry, physics, and biology. It aids in explaining chemical reactions, bonding, and the properties of materials.
In conclusion, Class 9 Science Chapter 4 – Structure of the Atom provides a crucial foundation for understanding the basic building blocks of matter. By exploring the various models of atomic structure, from Dalton’s theory to Bohr’s quantized orbits, students gain a comprehensive understanding of how atoms are composed and function. The chapter also delves into the discovery of subatomic particles, the concept of isotopes, and electron configuration, all of which are essential for further studies in chemistry and other scientific fields. With the Structure of the Atom as a key chapter, students are well-prepared to tackle more complex concepts in future lessons. iPrep’s resources for Class 9 Science Chapter 4 – Structure of the Atom ensure that you can master these concepts with engaging videos, practice questions, and detailed notes. Dive deeper into the Structure of the Atom and enhance your learning with iPrep!
Practice questions on Chapter 4 - Structure of the Atom
Get your free Chapter 4 - Structure of the Atom practice quiz of 20+ questions & detailed solutions
Practice Now








