Classification of Elements and Periodicity in Properties – Complete Guide For Class 11 Chemistry Chapter 3
Welcome to iPrep, your Learning Super App. Our learning resources for Chapter 3, “Classification of Elements and Periodicity in Properties,” in Class 11 Chemistry are meticulously designed to ensure students gain a comprehensive understanding of this essential topic. These resources include detailed notes on the historical development of the periodic table, including Mendeleev’s contributions and the modern arrangement based on atomic numbers.
They cover periodic trends such as atomic radius, ionization energy, electron affinity, and electronegativity, providing clear explanations of how these properties change across periods and down groups. Additionally, students are provided with visual aids, such as color-coded periodic tables, to highlight key trends and relationships between elements. Practice questions and exercises are included to reinforce learning and help students apply concepts to solve problems related to element properties and reactivity.
The concept of “Classification of Elements and Periodicity in Properties” in Class 11 Biology delves into the foundational principles of chemical elements that make up living organisms. This includes an understanding of how elements like carbon, hydrogen, oxygen, and nitrogen are classified and how their periodic properties influence biological molecules and processes. By examining the unique characteristics and roles of these elements, students learn how chemical properties impact the structure and function of biomolecules such as proteins, nucleic acids, and carbohydrates. This exploration helps in understanding the biochemical foundations that drive cellular functions and overall organismal physiology.
What is the Classification of Elements and Periodicity in Properties?
The classification of elements and periodicity in properties is a fundamental concept in chemistry that helps us understand the organization of the periodic table and the behavior of various elements. By categorizing elements based on their atomic structure and chemical properties, we can predict their reactivity, interactions, and trends within the table. This classification of elements not only simplifies the study of elements but also provides a systematic approach to analyzing their relationships with one another.
Understanding these principles is crucial for students, as it lays the foundation for further exploration of chemical reactions, bonding, and the properties of compounds formed by these elements. In this section, we will delve deeper into the significance of this classification and how it aids in comprehending the intricate world of chemistry.
Historical Development of the Periodic Table
The periodic table, as we know it today, was developed in 1869. Within that, the classification of elements is done based on increasing atomic numbers, allowing us to observe recurring sets of properties.
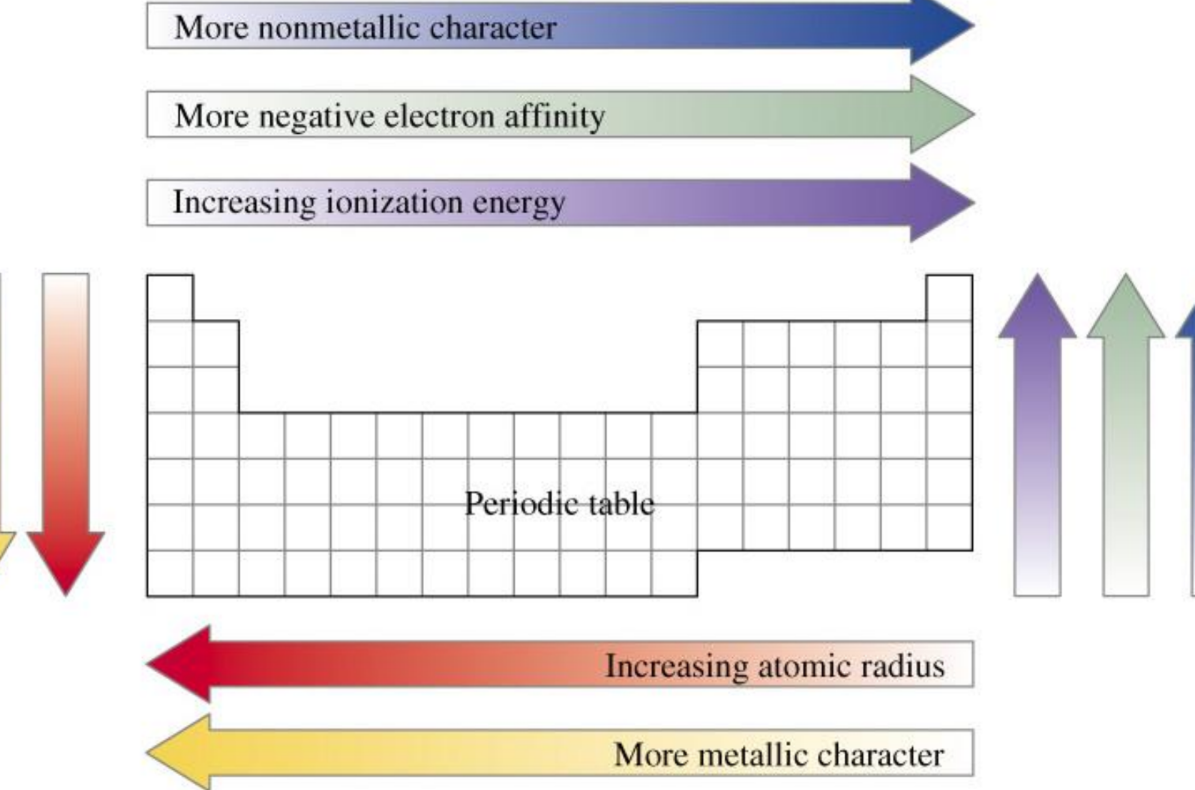
Periodic Trends in Properties
When the classification of elements is arranged in order of increasing atomic number, certain properties recur periodically. These properties include:
- Metallic, Nonmetallic, and Metalloid Properties: Elements exhibit distinct metallic, nonmetallic, or metalloid characteristics based on their position in the periodic table.
- Atomic Radius: The atomic radius is the distance from the nucleus to the outer boundary of the electron cloud. This property varies across periods and groups.
- Ionization Energies: Ionization energy is the energy required to remove the outermost electron from an atom. It varies across periods and groups in predictable ways.
- Electron Affinities: Electron affinity refers to the energy change when an electron is added to a neutral atom. This property also follows periodic trends.
- Reactivity: The reactivity of elements depends on their tendency to lose or gain electrons, which is influenced by their position in the periodic table.
- Electronegativity: Electronegativity measures an atom’s ability to attract and hold onto bonding electrons.
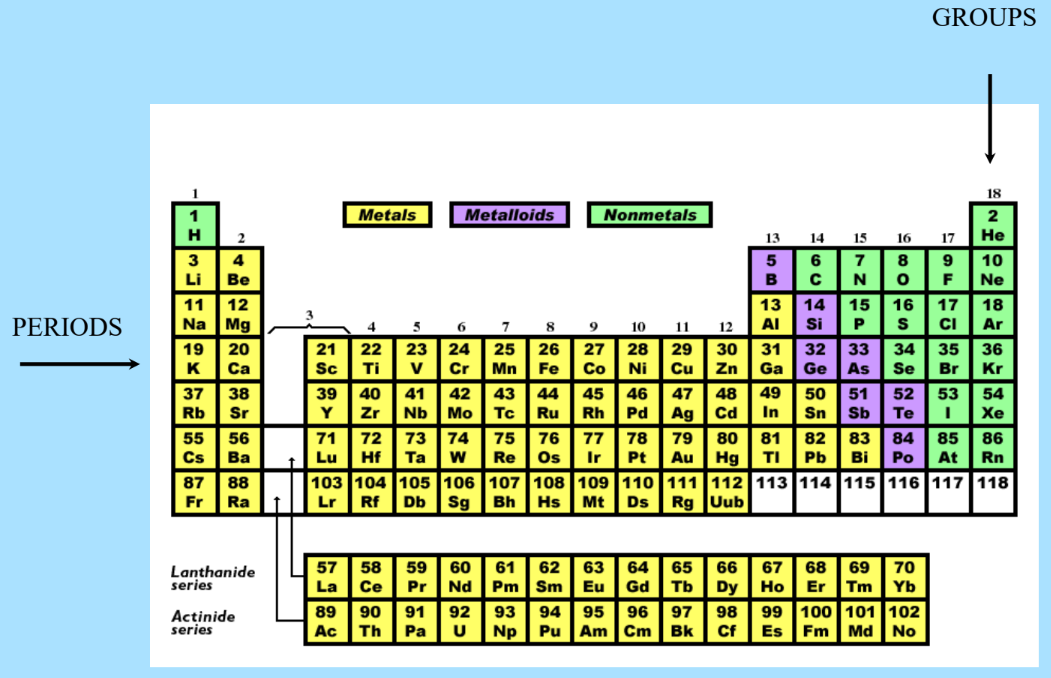
Modern Periodic Table
The modern periodic table designed for the classification of elements is organized into groups and periods:
- Groups: Vertical columns in the periodic table, where elements have similar chemical properties due to the same number of electrons in their outer energy levels.
- Periods: Horizontal rows in the periodic table. Elements in the same period have the same number of atomic orbitals.
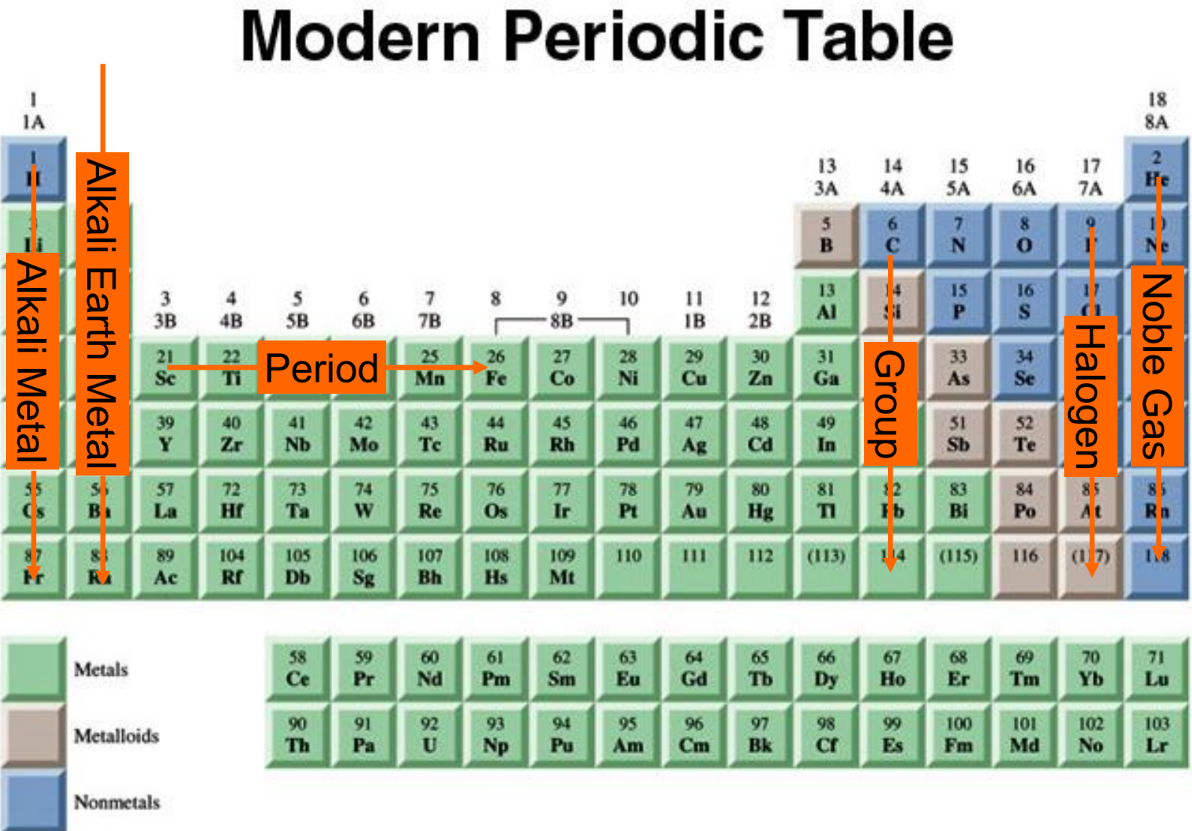
Element Families
Further in classification of elements, it mentioned that, elements can be divided into various families based on their properties:
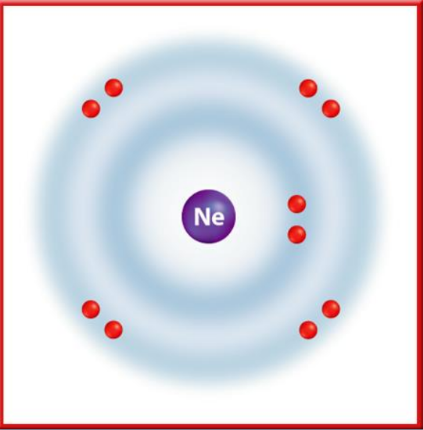
- Noble Gases (Group 18): Elements such as Neon have eight electrons in their outer energy levels, making them stable and unreactive.
- Halogens (Group 17): Elements like Fluorine are highly reactive nonmetals due to their high electronegativity and tendency to gain electrons.
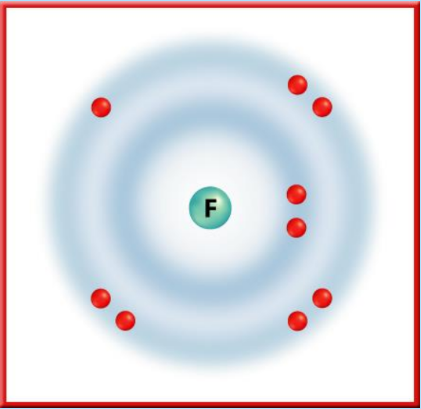
- Alkali Metals (Group 1): These elements, including Sodium and Potassium, are highly reactive metals with one outer energy level electron that is easily lost during chemical reactions. Reactivity increases down the group.
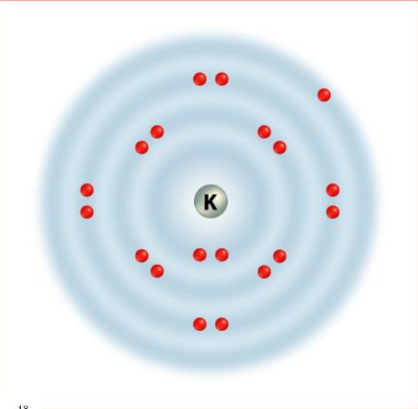
Trends in Atomic Size
The atomic size or atomic radius changes across periods and groups:
- Across a Period: Atomic radius decreases from left to right due to increasing nuclear charge, which pulls the electron cloud closer to the nucleus.
- Down a Group: Atomic radius increases because additional electron shells are added, making the atom larger.
Choosing the Larger Atom in Each Pair
- C or O: Carbon has a larger atomic radius than Oxygen.
- Li or K: Potassium has a larger atomic radius than Lithium.
- C or Al: Aluminum has a larger atomic radius than Carbon.
- Se or I: Iodine has a larger atomic radius than Selenium.
Variation of Atomic (Molar) Volume within Each Period
When practicing the classification of elements, atomic volume varies within each period, influenced by the atomic radius and the electron configuration of the elements.
Types of Atomic Radii
- Van der Waals Radius: Half the minimum distance between the nuclei of two atoms not bound to the same molecule.
- Ionic Radius: The radius of an atom forming an ionic bond. This radius differs for cations and anions due to size differences between the atoms forming the bond.
- Covalent Radius: Half the distance between the nuclei of two atoms bonded by a covalent bond, representing the radius of each atom.
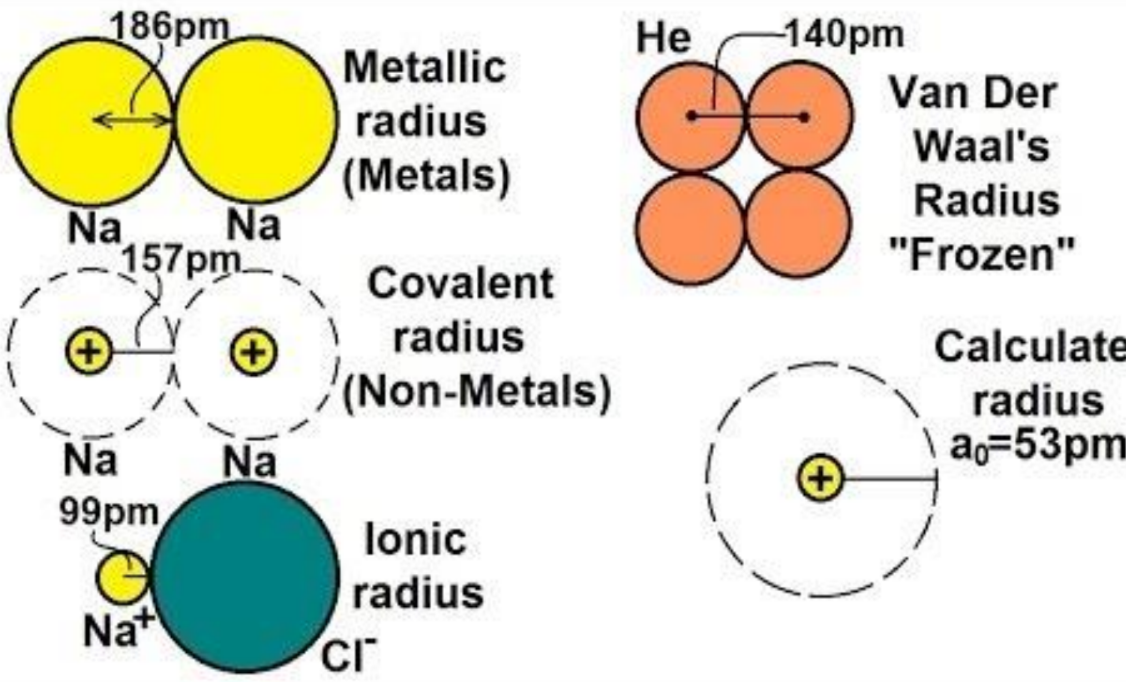
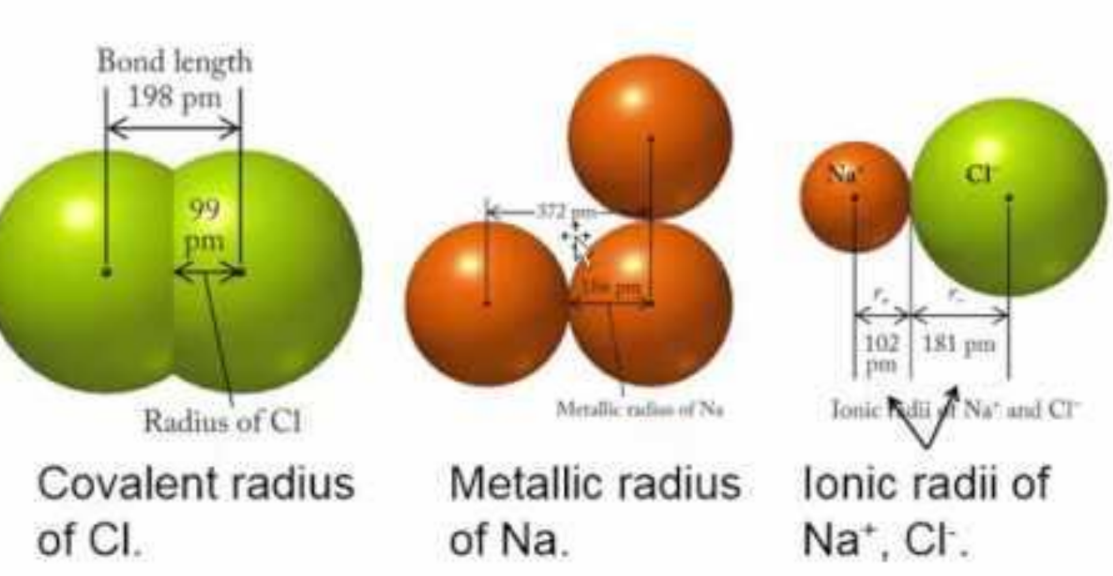
Relationship Between Different Radii
- Van der Waals Radius > Metallic Radius > Covalent Radius > Ionic Radius
Ionization Energies of Elements
Ionization energy is a key periodic property that decreases down a group and increases across a period. It reflects the energy needed to remove an electron from an atom, influencing reactivity.
Electron Affinity
Electron affinity is the energy change when an electron is added to a neutral atom. Generally, a more negative electron affinity indicates a more favorable process. It varies periodically, with nonmetals typically having more negative electron affinities than metals.
Electronegativity
Electronegativity measures an atom’s ability to attract a bonding pair of electrons. The Pauling scale is commonly used, with Fluorine as the most electronegative element. Electronegativity decreases down a group and increases across a period.
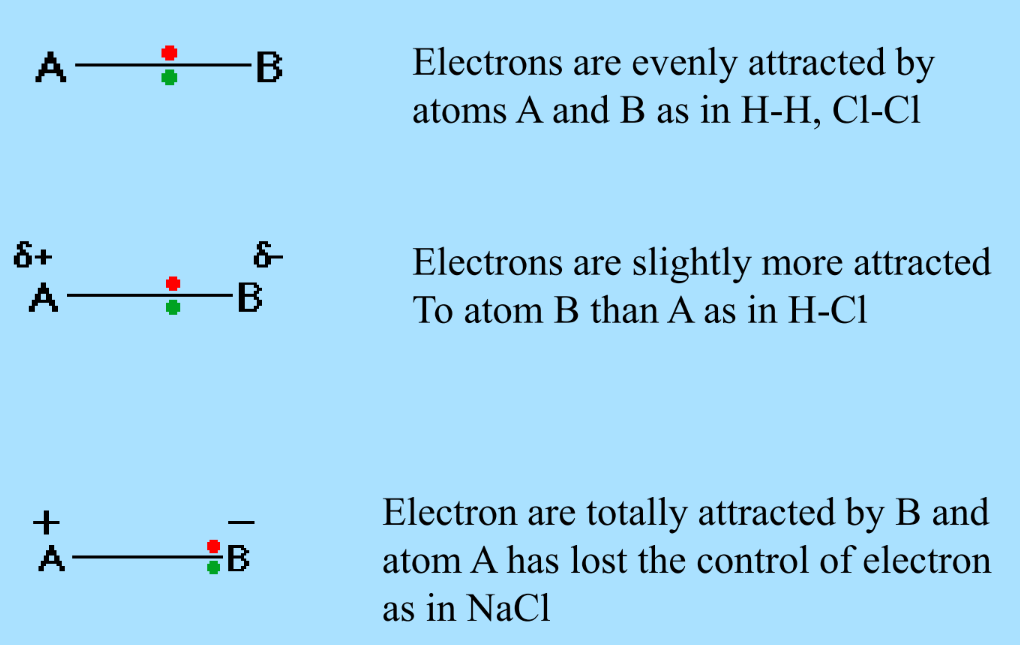
Examples of Electronegativity
- Equal Attraction: H-H, Cl-Cl
- Slight Attraction Difference: H-Cl
- Total Electron Transfer: NaCl
Other Periodic Properties
Several other properties also exhibit periodic trends within classification of elements:
- Melting and Boiling Points: Vary within a group and across a period due to differences in atomic structure and bonding.
- Conductivity: The ability to conduct heat and electricity changes across periods, with metals being good conductors and nonmetals being poor conductors.
- Reducing and Oxidizing Abilities: These properties vary within a group and are influenced by the element’s position in the periodic table.
- Acid-Base Nature of Element Oxides: Elements form oxides with varying acid-base characteristics depending on their position in the periodic table.
Conclusion
In summary, our guide on “Classification of Elements and Periodicity in Properties” serves as a valuable resource for CBSE Class 11 Chemistry students, equipping them with the knowledge needed to grasp essential concepts in this crucial chapter. By exploring the historical context of the periodic table and understanding the significance of atomic structure, students can appreciate how elements are organized based on their properties.
The periodic trends discussed, such as variations in atomic radius, ionization energy, and electron affinity, further illustrate how these factors affect the reactivity and relationships between different elements. Mastery of the “Classification of Elements and Periodicity in Properties” chapter not only lays the groundwork for advanced studies in chemistry but also fosters a deeper appreciation for the role that these fundamental concepts play in the broader scientific landscape. We encourage students to engage with the provided resources, practice questions, and visual aids to solidify their understanding of this foundational topic in chemistry.
Practice questions on Chapter 3 - Classification Of Elements
Get your free Chapter 3 - Classification Of Elements practice quiz of 20+ questions & detailed solutions
Practice Now








