Physical and Chemical Changes – Complete Guide For Class 7 Science Chapter 5
Welcome to iPrep, your Learning Super App. Our learning resources for the chapter, Physical and Chemical Changes in Science for Class 7th are designed to ensure that you grasp this concept with clarity and perfection. Whether you’re studying for an upcoming exam or strengthening your concepts, our engaging animated videos, practice questions and notes offer you the best of integrated learning with interesting explanations and examples.
Welcome to our in-depth exploration of Chapter 5 Physical and Chemical Changes from Science Class 7, where we dive into the fascinating world of physical and chemical changes. Understanding these changes helps us appreciate the dynamic nature of matter and its transformations.
Let’s break down the concepts and examples to make these scientific principles clear and engaging.
What is a Physical Change?
The chapter on physical and chemical changes starts with a clear explanation of what is a physical change. It involves a change in the physical properties of a substance. These properties include color, shape, size, and state (solid, liquid, gas). Importantly, during a physical change, no new substances are formed.
Let’s explore some of the key characteristics of a physical change.
Key Characteristics of Physical Changes
The chapter on physical and chemical changes further covers key characteristics of physical changes. Those involve:
- Reversible: Most physical changes can be reversed.
- No New Substances: No new substances are created.
- Observable Properties: Changes in size, shape, color, or state.
Here are some examples of physical change taking place in our daily lives.
Examples of Physical Changes
| Example | Description |
| Freezing of Water | Liquid water turns into solid ice at 0 °C. |
| Evaporation of Water | Water turns into vapor when heated to 100 °C. |
| Melting of Wax | Solid wax becomes liquid when heated. |
| Crumpling a Paper | Shape changes but paper remains paper. |
| Bending of Wire | The foil is torn into smaller pieces. |
| Cutting Wood | Wood is cut into smaller pieces, but it remains wood. |
| Ripping Foil Paper | Foil is torn into smaller pieces. |
| Crushing a Can | A can’s shape is distorted but it remains aluminum. |
Some detailed examples of Physical Changes
- Freezing of Water:
Freezing is when liquid water turns into a solid (ice) at or below 0 °C. The freezing point is a crucial concept where the phase transition occurs. - Evaporation of Water:
This process transforms liquid water into vapor when heated to 100 °C, known as the boiling point. Other factors like humidity and sunlight also influence evaporation. - Melting of Wax:
Melting is the transformation of solid wax into liquid when subjected to higher temperatures, such as when burning a candle.
Look at the diagram given below to understand different types of physical transitions taking place between various states of matter.
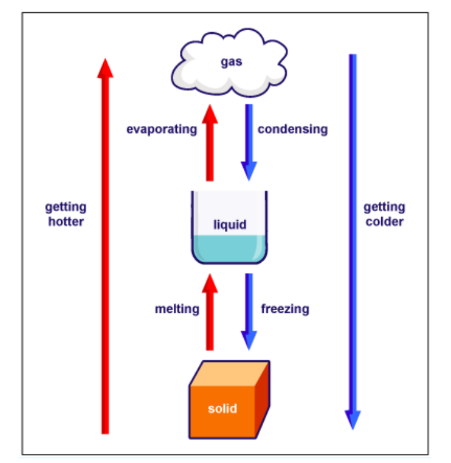
What is a Chemical Change?
A chemical change results in the formation of new substances through the transformation of one or more substances. This process is also known as a chemical reaction.
Let’s explore some of the key characteristics of a chemical change.
Key Indicators of Chemical Changes
- New Substances Formed
- Sound, Light, or Heat Produced
- Color Change
- Gas Formation
- Smell
Let’s explore some examples of chemical change taking place in our daily lives.
Examples of Chemical Changes
| Example | Resulting Products |
| Vinegar + Baking Soda | Carbon dioxide + Other substances |
| Burning of Wood | Heat, light, ash, and other substances |
| Iron + Water + Oxygen | Rust (iron oxide Fe₂O₃) |
Here are some detailed examples of chemical changes.
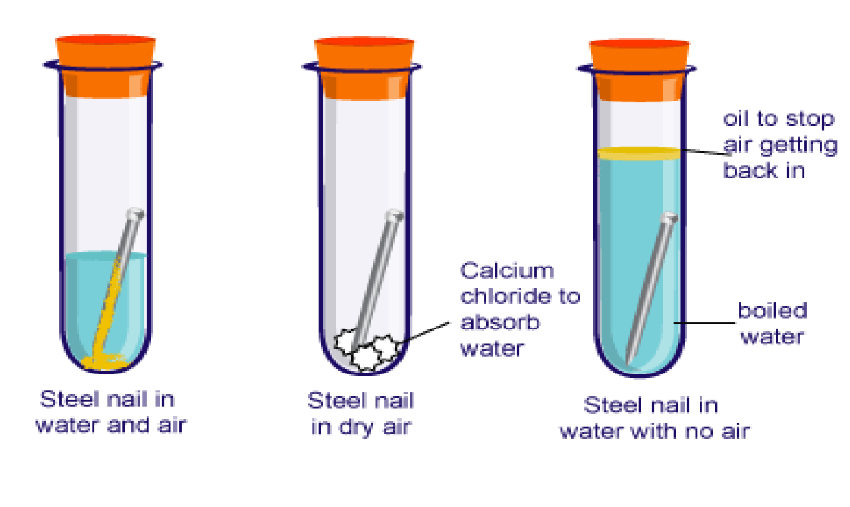
Rusting
- Definition: Rust is the brownish film that forms on iron due to the reaction with oxygen in the presence of water or air moisture.
- Rusting Equation: Iron (Fe) + Oxygen (O₂) + Water (H₂O) → Rust (Fe₂O₃)
- Prevention:
- Avoid exposure to moisture and oxygen.
- Use coatings like paint or grease.
- Apply a layer of another metal such as zinc (galvanization).
Galvanisation
- Process: Depositing a protective layer of another metal like zinc or chromium on iron to prevent rusting.
- Examples: Galvanized iron pipes and car frames.
Stainless Steel
- Composition: Contains iron, carbon, chromium, nickel, and manganese. It is resistant to corrosion.
Crystallization
- Definition: The formation of solid crystals from a solution.
- Examples:
- Salt from seawater
- Snowflakes
- Honey crystallization
- Sucrose crystallization from aqueous solutions
Let’s look at the comparison between physical and chemical changes with some more practical examples.
Comparative Table of Examples
| Physical Changes | Chemical Changes |
| Foil is cut into small pieces. | Milk becomes sour. |
| Clay is molded into a new shape. | Jewelry darkens. |
| Ice melts into water. | Bread becomes toast. |
| Water evaporates from a river. | Rust forms on a nail. |
Conclusion
Understanding the distinctions between physical and chemical changes from CBSE class 7 science chapter 5 helps us understand how substances interact and transform our everyday lives. Whether it’s wax melting or iron rusting, each process has unique characteristics and implications. We hope this guide clarifies these concepts and provides valuable insights into the fascinating world of matter and its changes.
Practice questions on Chapter 5 - Physical and Chemical Changes
Get your free Chapter 5 - Physical and Chemical Changes practice quiz of 20+ questions & detailed solutions
Practice Now








