Chemical Effects of Electric Current – Complete Guide For Class 8 Science Chapter 11
Welcome to iPrep, your Learning Super App! Our learning resources for the chapter, Chemical Effects of Electric Current Class 8th chapter 11 are designed to ensure that you grasp this concept with clarity and perfection. Whether you’re studying for an upcoming exam or strengthening your concepts, our engaging animated videos, practice questions and notes offer you the best of integrated learning with interesting explanations and examples.
The chapter on the Chemical Effects of Electric Current in Class 8 Science introduces students to the fundamental concept that electric current can induce chemical changes when passed through a conducting solution. This phenomenon explains various practical applications, such as electroplating, electrolysis, and the functioning of batteries. By exploring these chemical effects, students gain an understanding of how electricity can drive chemical reactions and its significance in industrial processes and everyday technology. This knowledge lays the foundation for future studies in both chemistry and physics, highlighting the interdisciplinary nature of scientific principles.
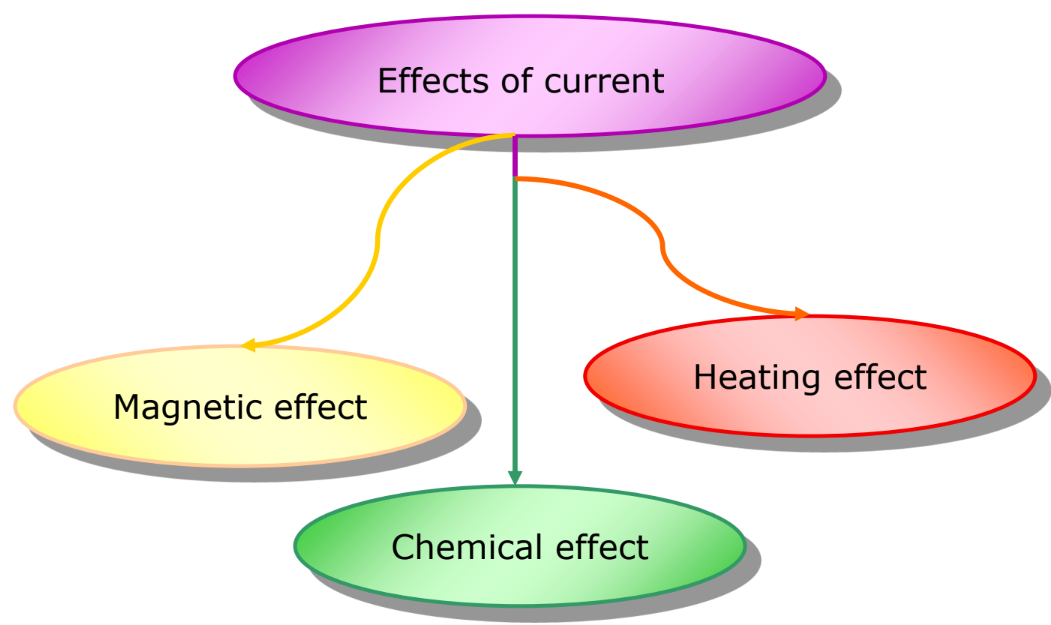
Effects of Electric Current
Electric current can have several effects, including:
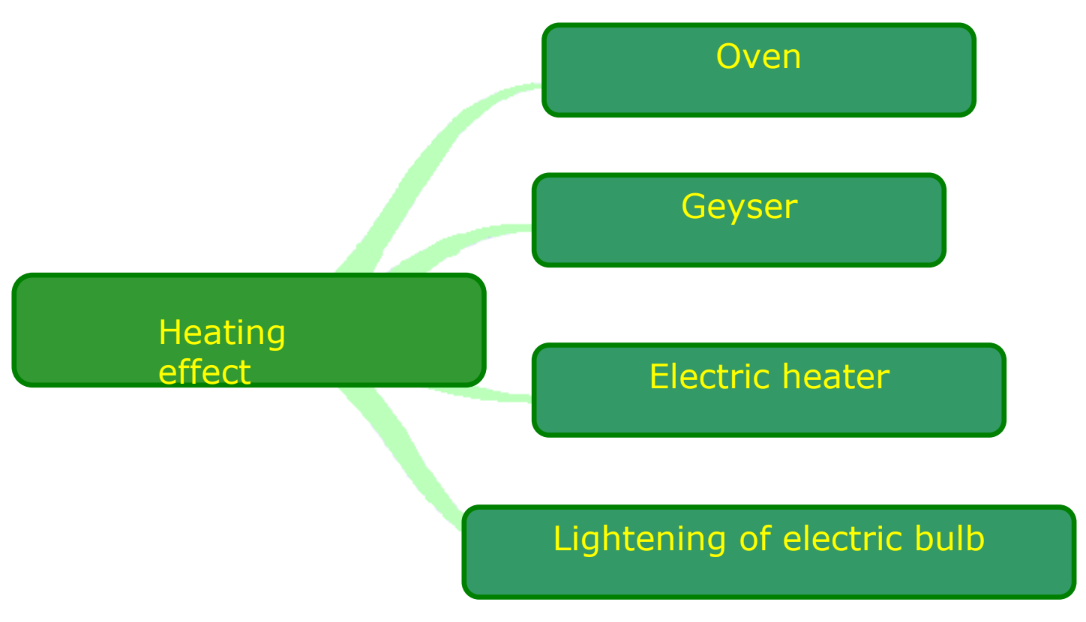
- Heating Effect of Current: Observed in devices like electric heaters and ovens.
- Magnetic Effect of Current: Utilized in devices such as loudspeakers, ammeters, and electric motors.
- Chemical Effect of Current: This is the primary focus of this chapter.
Chemical Effects of Electric Current
The chemical effects of current involve the chemical transformations that happen when an electric current flows through a conducting solution or electrolyte. These changes can include the decomposition of compounds, deposition of materials, or liberation of gases. This phenomenon is utilized in various applications, such as electroplating and electrolysis.
Essential Components and Devices – These chemical effects of current are fundamental to the operation of crucial components and devices such as:
- Voltmeters and Ammeters: These are used to measure voltage and current, respectively.
- Electrodes:
- Anode: The positive electrode.
- Cathode: The negative electrode.
Let’s Get Ready for an Experiment on the Chemical Effects of Current!
Materials Needed
- Discarded cells
- Sandpaper
- Copper wires
- Battery
Simple Steps
- Extract carbon rods from the cells.
- Clean the metal caps with sandpaper.
- Wrap copper wires around the metal caps.
- Connect them to a battery.
Light Emitting Diode (LED): The functioning of Light Emitting Diodes (LEDs) is based on the principles of electroluminescence, which involves the chemical effects of current.
- LED: A device that emits light when an electric current passes through it.

- Usage: Commonly used as indicator lights due to their efficiency and low power consumption.
Conductors and Insulators
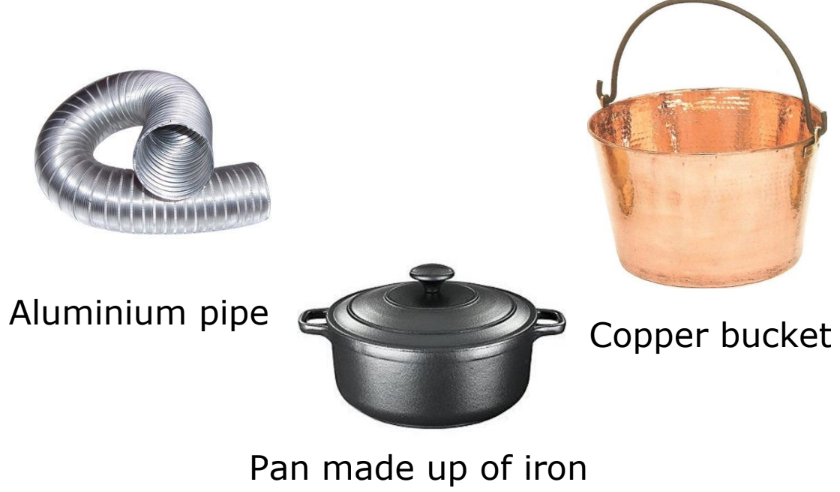
- Conductors: Materials that allow electric current to pass through them easily (e.g., aluminum, iron, copper).
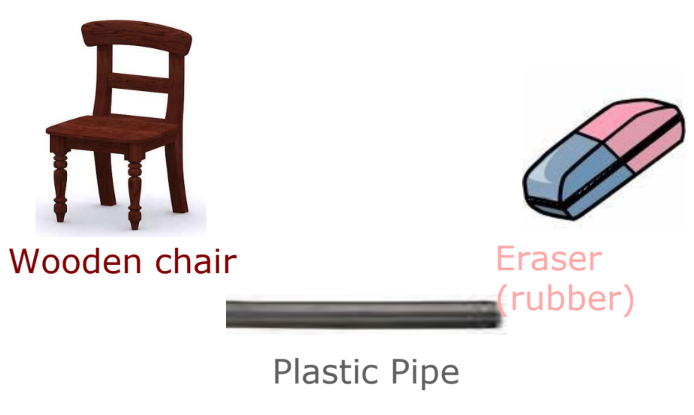
- Insulators: Materials that do not allow electric current to pass through them easily (e.g., rubber, plastic, wood).
Testing Materials
To determine if a material is a conductor or an insulator, you can use a simple tester:
- Make a Circuit: Connect an electric cell, a bulb, a safety pin, and connection wires.
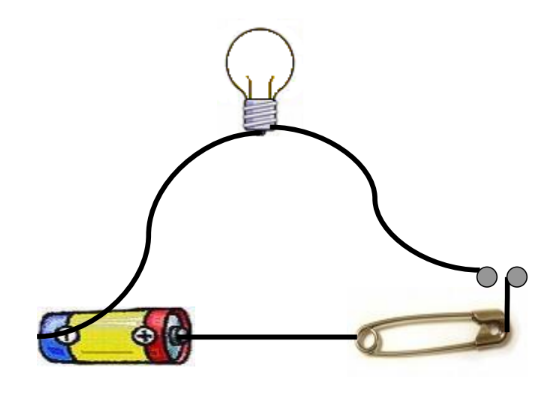
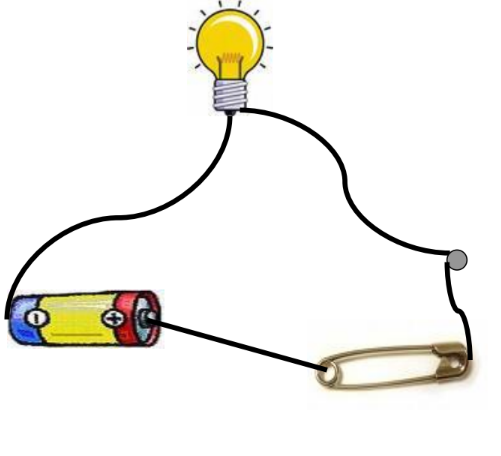
- Check the Tester: Ensure there are no loose connections, the bulb is not fused, and the batteries are charged.
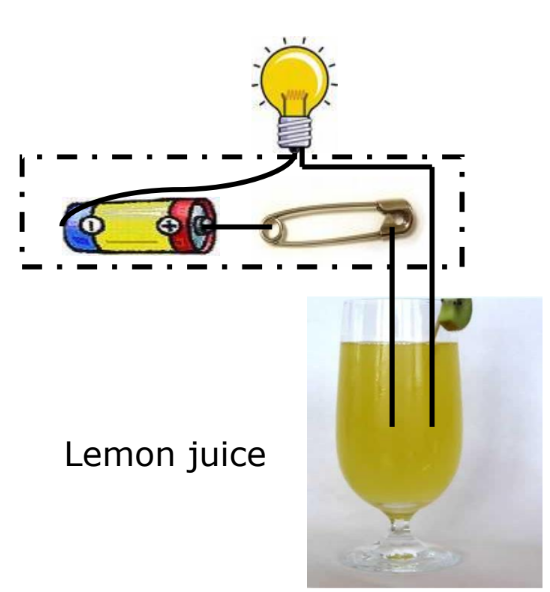
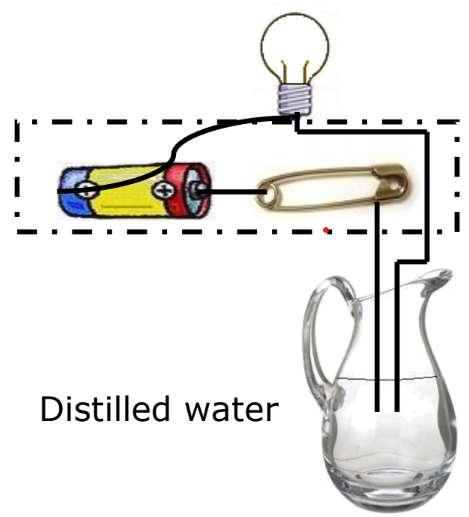
Do Liquids Conduct Electricity? When an electric current passes through a liquid, it can cause chemical changes depending on the liquid’s conductivity.
Experiment
- Materials: Lemon juice, distilled water.
- Observation:
- If the bulb glows, the liquid conducts electricity.
- If the bulb does not glow, the liquid does not conduct electricity.
Conductive Liquids:
- Acidic solutions
- Basic solutions
- Salt solutions
Applications of the Chemical Effect of Current
Electroplating
Electroplating involves coating one metal with another using electric current. This process is widely used in making jewelry and providing corrosion protection.
Electrolysis
Electrolysis is a process where an electric current passes through an electrolyte, causing a chemical change.
Electroplating: Applying the Chemical Effect of Current
Materials Needed
- Two copper plates
- Copper sulphate (2 teaspoons)
- Dilute sulphuric acid (a few drops)
- Distilled water (250 mL)
- Beaker
- Battery
Steps
- Take distilled water in a beaker.
- Dissolve copper sulfate in the water.
- Add dilute sulphuric acid.
- Connect the copper plates to the battery terminals.
- Immerse the plates in the copper sulfate solution and allow the current to pass for 15 minutes.
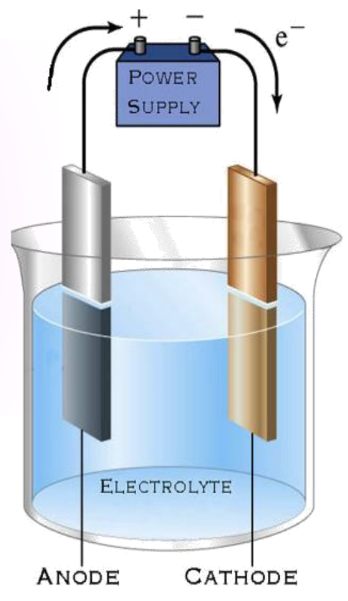
Observation:
- Copper sulfate dissociates into copper and sulfate.
- Free copper deposits on the electrode are connected to the negative terminal.
Identification of Chemical Reactions
When electric current passes through a conducting solution, it can cause:
- Formation of gas bubbles on the electrodes.
- Deposition of metal on the electrodes.
- Change in the color of the solution.
Uses of Electroplating
- Jewelry Making: Provides a beautiful and durable finish.

- Corrosion Protection: Helps protect metal surfaces from rust and corrosion.
This chapter provides a comprehensive understanding of the chemical effects of current and its practical applications, making it a vital part of the Class 8 Science curriculum. Students will explore how electric currents induce chemical changes, leading to various industrial and everyday applications such as electroplating, electrolysis, and battery operation. Through hands-on experiments and real-world examples, this chapter not only enhances their theoretical knowledge but also demonstrates the practical significance of these chemical processes, fostering a deeper appreciation for the interconnectedness of science and technology.
Practice questions on Chapter 11 - Chemical Effects of Electric Current
Get your free Chapter 11 - Chemical Effects of Electric Current practice quiz of 20+ questions & detailed solutions
Practice Now








