Metals and Non-Metals – Complete Guide For Class 10 Science Chapter 3
Welcome to iPrep, your Learning Super App. Our learning resources for the chapter, Metals and Non-Metals in Science for Class 10th are designed to ensure you grasp this concept with clarity and perfection. Whether you’re studying for an upcoming exam or strengthening your concepts, our engaging animated videos, practice questions and notes offer you the best of integrated learning with interesting explanations and examples.
Get ready to explore one of the most fascinating chapters in your CBSE Class 10th Science curriculum – Chapter 3: Metals and Non-Metals. This chapter delves deep into the unique characteristics, behaviors, and reactions of metals, non-metals, and metalloids. From understanding how metals conduct electricity to learning why non-metals are brittle, Chapter 3 – Metals and Non-Metals will equip you with essential knowledge about the elements that make up our world. Through detailed examples and practical applications, you’ll discover how these materials shape industries, technology, and everyday life.
Understanding the fundamental differences between metals, non-metals, and metalloids is crucial for comprehending their unique properties and applications. Let’s dive into the details of this chapter to get a clear picture starting with the question of the hour.
What are Metals and Non-Metals?
Metals and non-metals are two fundamental groups of elements, each with distinct physical and chemical properties that define their role in nature and industry. Let’s understand them one by one.
Metals
Definition and Characteristics
- Metals have 1, 2, or 3 electrons in their valence shell.
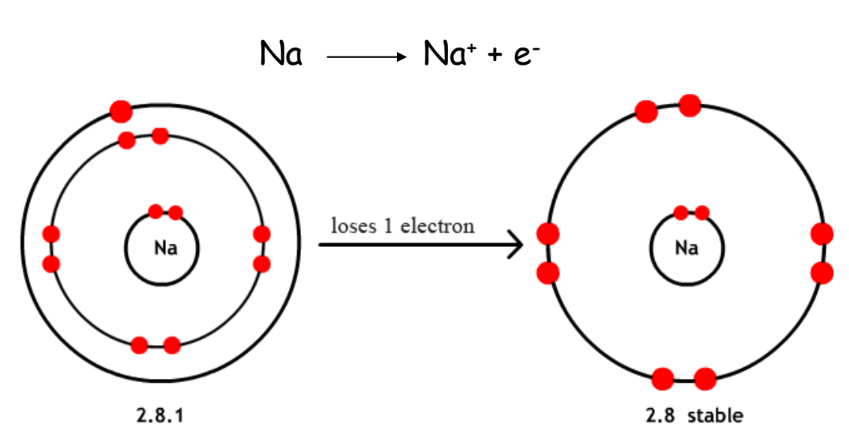
- Example: Iron (Fe), Sodium (Na), Aluminium (Al).
- Sodium (Na) electronic configuration: 2, 8, 1.
- Metals form positive ions by losing electrons.
Physical Properties of Metals
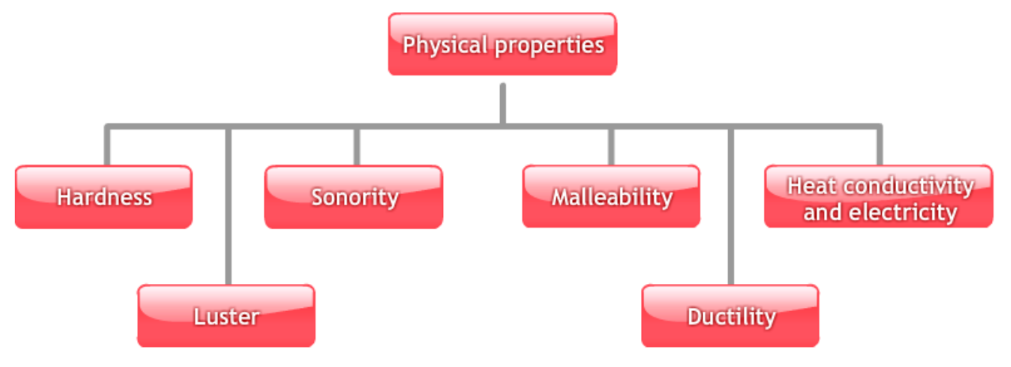
As per the chapter metals and non-metals, here are the physical properties of metals-
- Hardness: Most metals are hard, except sodium and potassium which can be cut with a knife.
- Luster: Freshly cut metals have a shiny surface known as metallic luster.
- Malleability: Metals can be beaten into sheets.
- Ductility: Metals can be drawn into wires.
- Conductivity: Metals are good conductors of heat and electricity.
- Sonority: Metals produce a musical sound when struck.
Chemical Properties of Metals
As per the chapter metals and non-metals, here are the chemical properties of metals-
- Reaction with Oxygen: Metals react with oxygen to form metal oxides.
- Most metal oxides are basic.
- Some, like oxides of zinc and aluminum, are amphoteric.
- Reaction with Water: Metals react with water to form metal hydroxides or oxides and hydrogen gas.

- Only metals above hydrogen in the activity series react with water.
- Na, K, and Ca react vigorously with water.
- Mg reacts with hot water.
- Al and Fe react with steam.
- Reaction with Acids: Metals react with dilute acids to produce metal chlorides and hydrogen gas.
- Only metals above hydrogen in the activity series give this reaction.
- Dilute nitric acid does not give this reaction as it is an oxidizing agent.
- Dilute nitric acid, however, reacts with Mg and Mn to evolve hydrogen gas.
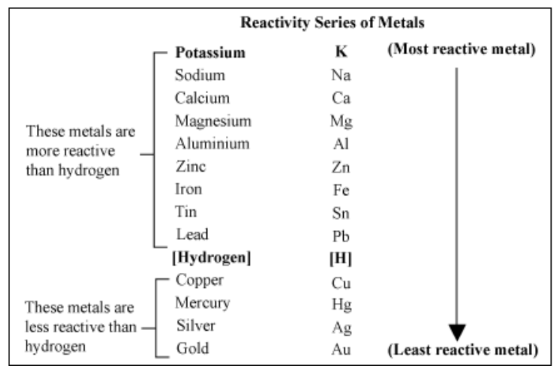
Reactivity Series
As stated in the chapter Metals and Non-metals, here’s the reactivity series of metals-
Metals are arranged in the reactivity series based on their ability to displace other metals from their salt solutions.

Let’s understand different types of reactions one by one.
Displacement Reactions
- A more reactive metal displaces a less reactive metal from its salt solution.
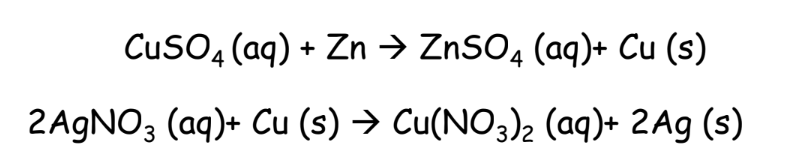
Non-Metals
Definition and Characteristics
- Non-metals have 5, 6, or 7 electrons in their valence shell.
- Example: Sulphur (S), Chlorine (Cl).
- Chlorine (Cl) electronic configuration: 2, 8, 7.
- Non-metals form negative ions by gaining electrons.

Physical Properties of Non-Metals
As per the chapter metals and non-metals, here are the physical properties of non-metals-
- Brittleness: Non-metals are brittle, except diamonds.
- Lustre: Non-metals are non-lustrous and cannot be polished, except iodine.
- Malleability and Ductility: Non-metals are neither malleable nor ductile.
- Conductivity: Non-metals are thermal and electrical insulators.
- Sonority: Non-metals do not produce a musical sound when struck.
Chemical Properties of Non-Metals
- Reaction with Oxygen: Non-metals react with oxygen to form oxides. Most of the non-metal oxides are ACIDIC or NEUTRAL in nature.
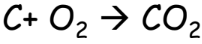
- Most non-metal oxides are acidic or neutral.
- Reaction with Acids: Non-metals do not react with dilute acids to produce hydrogen gas. It is so because non-metals are electron acceptors so they cannot reduce H+ ions furnished by acids and liberate hydrogen gas.
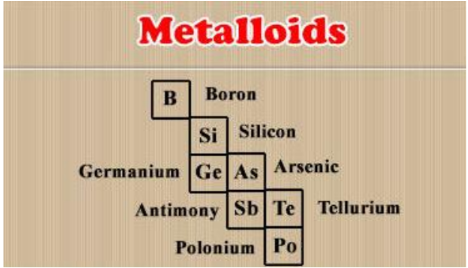
Metalloids
Definition and Characteristics
- Metalloids share properties of both metals and non-metals.
- They have 4 valence electrons.
- Example: Silicon (Si).
Reaction Between Metals and Nonmetals
- Metals donate electrons while non-metals accept electrons, forming ionic compounds.
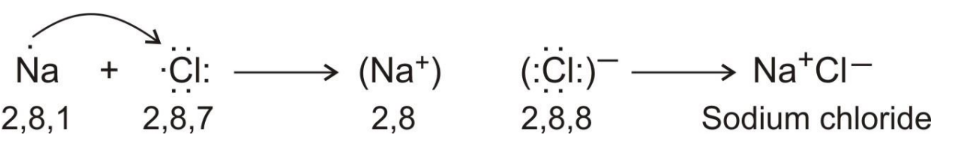
Ionic Bond
- Definition: A chemical bond formed by the transfer of electrons from one atom to another.
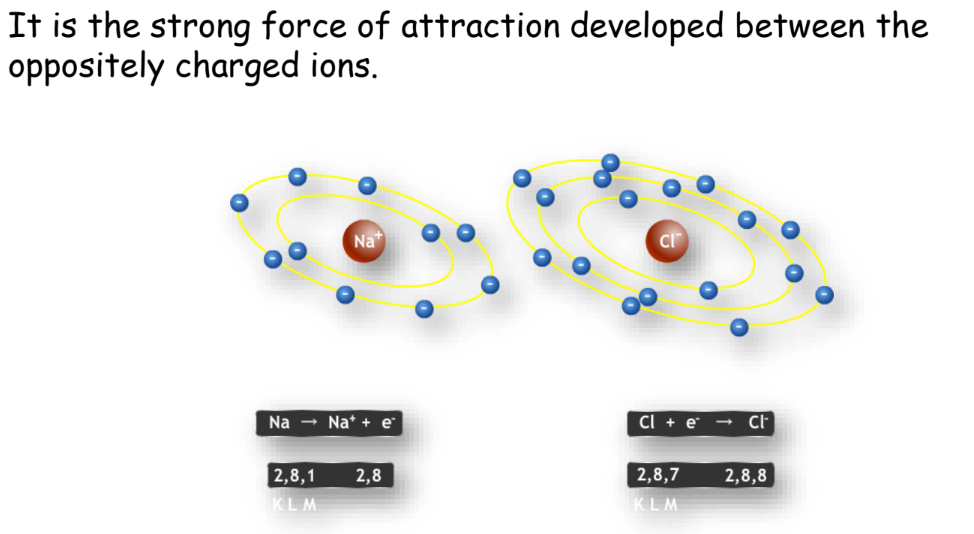
- Properties of Ionic Compounds:
- Crystalline solids.
- High melting and boiling points.
- Soluble in water but insoluble in organic solvents.
- Conduct electricity when dissolved in water or melted.
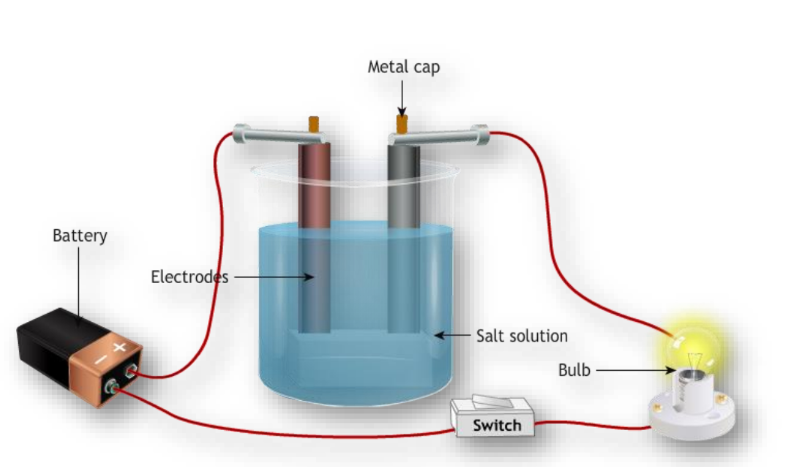
Occurrence and Extraction of Metals
Occurrence in Nature
- Most metals occur in a combined state.
- Metals like silver, gold, and platinum occur in a free state.
- Minerals: Metal-bearing substances in the earth’s crust.
- Ores: Minerals from which metals can be profitably extracted.
- Example: Zinc blende (ZnS), Galena (PbS).
Metallurgy
- Definition: The process of extracting metals from their ores and refining them.

- Steps in Metallurgy:
- Concentration or Enrichment of Ore:
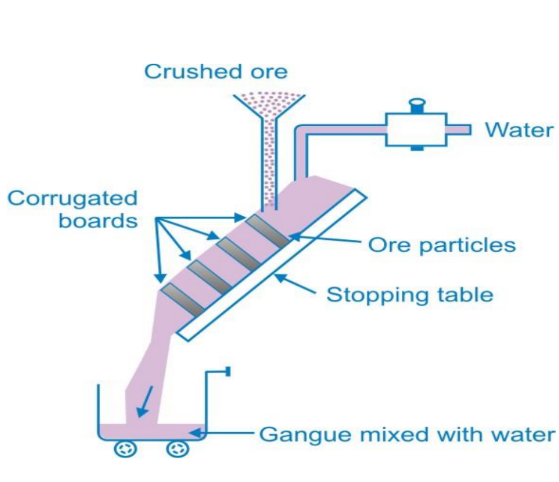
- Hydraulic Washing: Used when impurities are denser than ore particles.
- Concentration or Enrichment of Ore:
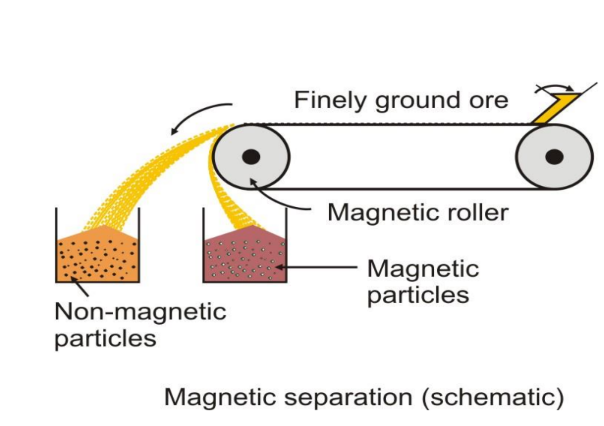
- Magnetic Separation: Used when either the ore or impurity is magnetic.
- Froth Flotation: Used for sulfide ores using pine oil as a frothing agent.
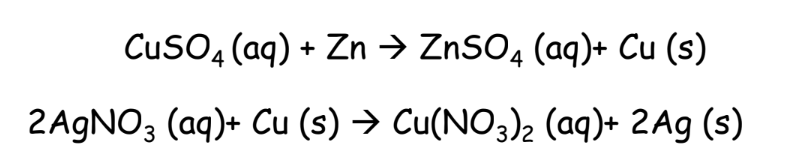
- Leaching: The powdered ore is treated with a suitable solvent. For Example, Bauxite ore is treated with NaOH solution for concentration.
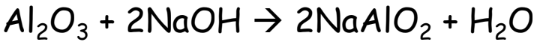
- Conversion of Enriched Ore to Metal Oxide: It is easy to extract metal from its oxide so the ore can be converted to metal oxide by:
- Calcination: Heating ore strongly in the absence of air.
- Roasting: Heating more strongly in the presence of air.

- Extraction of Metals from Metal Oxides:
- Reduction with Reducing Agent: Using coke, carbon monoxide, or hydrogen.
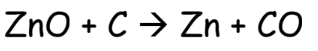
- Electrolytic Reduction: Used for active metals like Na, K, Mg, and Ca.
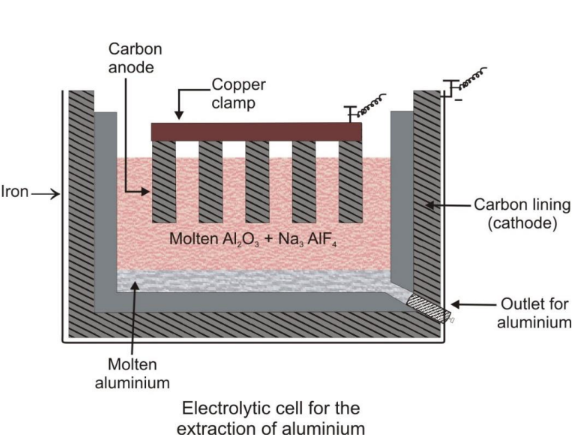
- Refining of Impure Metal:
- The metal obtained from the ore is not pure. It contains various substances as impurities.
- The process of removing these impurities is called refining.
- Methods include poling, electrolytic refining, and liquidation.
Alloys
- Definition: A homogeneous mixture of two or more metals or a metal and a non-metal in a definite proportion.
- Example: Brass (Copper + Zinc), Bronze (Copper + Tin).
Properties of Alloys
- Stronger and more resistant to corrosion than constituent metals.
- Lower melting point and electrical conductivity.
- Harder than the constituent metals.
Corrosion
- Definition: Formation of metal oxides on the surface of metals in the presence of air and water.
- Rusting: Corrosion of iron.
- Conditions for Rusting: Water and air both are essential. The presence of salts, acids, carbon dioxide, and sulfur dioxide in water enhances the process of rusting of iron.
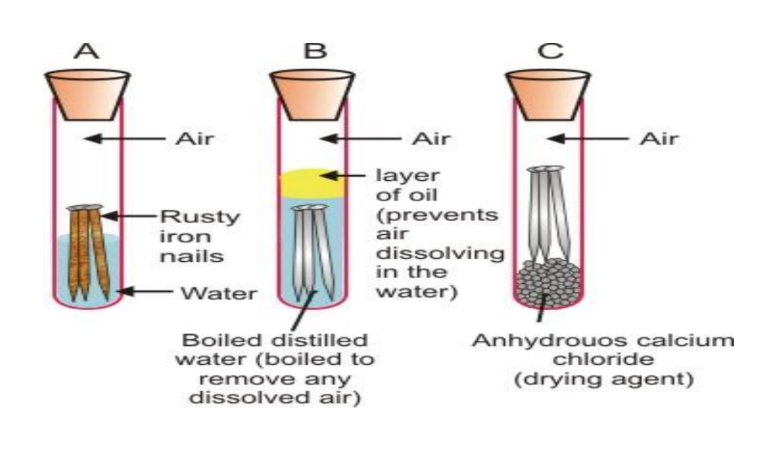
- Prevention Methods:
- Galvanization
- Electroplating
- Tin plating
- Painting
- Greasing
- Enameling
- Plastic coating
- Alloy formation
By understanding these concepts, students will be better prepared to grasp the intricate details and practical applications of metals and non-metals. Happy studying!
Let’s Conclude
In conclusion, CBSE Class 10th Science Chapter 3 – Metals and Non-Metals covers a wide array of concepts essential for understanding the characteristics and applications of metals, non-metals, and metalloids. From their physical and chemical properties to the extraction and uses of metals, this chapter provides a strong foundation in these critical areas of chemistry. The knowledge from Chapter 3 – Metals and Non-Metals will not only help you excel in your exams but also deepen your appreciation of the materials that shape the world around you. With iPrep’s resources, learning Chapter 3 – Metals and Non-Metals becomes an engaging and effective experience. Happy learning!
Practice questions on Chapter 3 - Metals and Non-Metals
Get your free Chapter 3 - Metals and Non-Metals practice quiz of 20+ questions & detailed solutions
Practice Now








