Carbon and Its Compounds – The Best Guide For Class 10 Science Chapter 4
Welcome to iPrep, your Learning Super App. Our learning resources for the chapter, Carbon and its Compounds in Science for Class 10th are designed to ensure that you grasp this concept with clarity and perfection. Whether you’re studying for an upcoming exam or strengthening your concepts, our engaging animated videos, practice questions and notes offer you the best of integrated learning with interesting explanations and examples.
Carbon is an integral part of our daily lives, present in various forms and compounds around us. From the bristles of our toothbrushes to the food we eat, carbon’s omnipresence is undeniable. This blog covers the detailed concepts of carbon and its compounds, their unique properties, and their significant role in chemistry.
The Objective of Learning Carbon and Its Compounds
CBSE Class 10th Science Chapter 4 – Carbon and Its Compounds delves into the remarkable element that forms the foundation of organic chemistry. Carbon’s unique ability to bond with itself and other elements leads to the creation of a vast array of compounds essential to both life and industry. From the structure of hydrocarbons to the chemistry behind soaps and detergents, this chapter explores how carbon compounds shape our world in fascinating ways. Understanding these concepts is crucial not only for your exams but also for appreciating the underlying science in everyday materials and processes.
What is Carbon?
As stated in the chapter carbon and its compounds Carbon is a versatile non-metal element with atomic number 6, known for its ability to form a vast number of compounds, making it the backbone of organic chemistry.
Atomic Number and Position in the Periodic Table
- Atomic Number: 6
- Group: 14 in the periodic table
- Nature: Non-metal
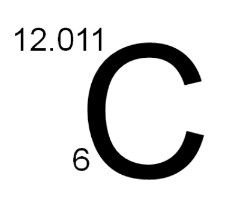
Carbon’s position in the periodic table is crucial as it is central to organic chemistry due to its ability to form a vast number of compounds with other elements like hydrogen, oxygen, and nitrogen.
Occurrence of Carbon
As stated in the carbon and its Compounds chapter, carbon is found in various forms on Earth:
- Earth’s Crust: 0.02% in the form of minerals (carbonates, hydrogen carbonates, coal, petroleum).
- Atmosphere: 0.03% in the form of carbon dioxide.
- Sea: Present as dissolved carbon dioxide.
- Living Matter: Composed of water and carbon compounds.
- Organic Matter: It is an essential component of all organic compounds (petrol, oil, hydrocarbons, etc.).
Organic Compounds and their Physical Properties
| Organic Compounds | Melting Point (K) | Boiling Point (K) |
| Methane | 90 | 111 |
| Chloroform | 209 | 334 |
| Ethanol | 156 | 351 |
| Acetic Acid | 290 | 391 |
These data suggest that the forces of attraction between molecules in organic compounds are not very strong. Additionally, these compounds are non-conductors of electricity, indicating that the bonding in these compounds does not produce ions.
Bonding in Carbon Compounds
As per the chapter carbon and its compounds, it is important to understand the bonding in the carbon compounds. Let’s break that down step by step-
Atomic Structure and Bonding
- Atomic Number: 6
- Electronic Configuration: 2, 4
- Valence Electrons: 4
Carbon can achieve a stable electronic configuration in two ways:
- Losing 4 electrons to resemble the helium gas configuration (2).
- Gaining 4 electrons to achieve the neon gas configuration (2, 8).
However, these methods are challenging due to energy requirements. Therefore, carbon shares electrons to form covalent bonds.
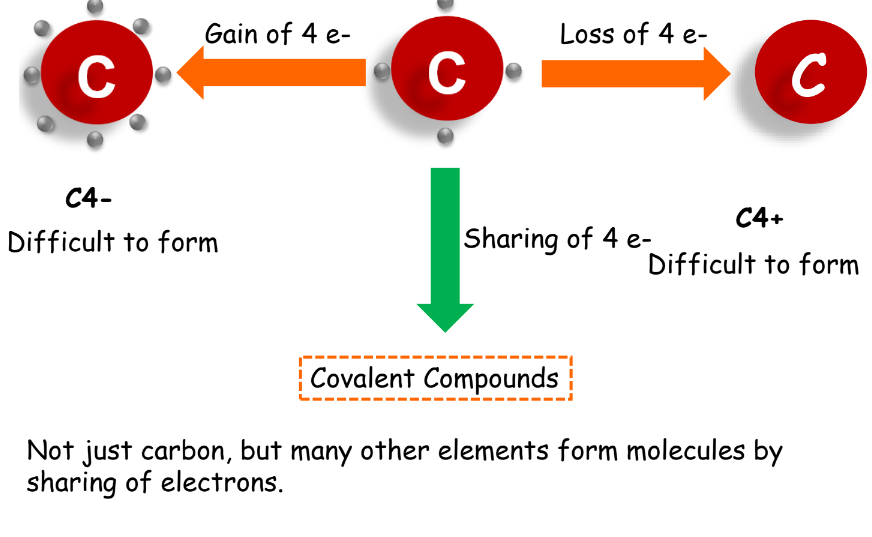
Covalent Bonds
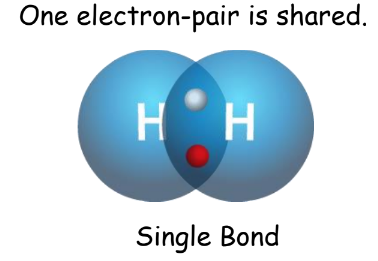
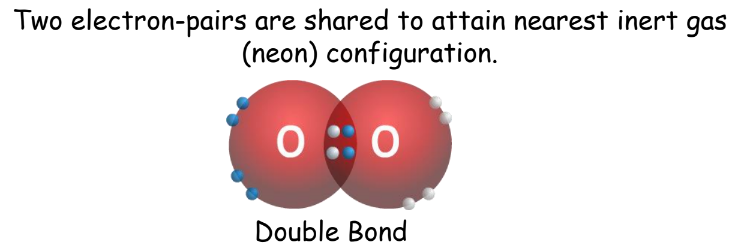
Covalent bonds are formed by the sharing of electrons between atoms. Based on the number of electron pairs shared, covalent bonds can be:
- Single Covalent Bond: One electron-pair shared.
- Double Covalent Bond: Two electron pairs shared.
- Triple Covalent Bond: Three electron pairs shared.
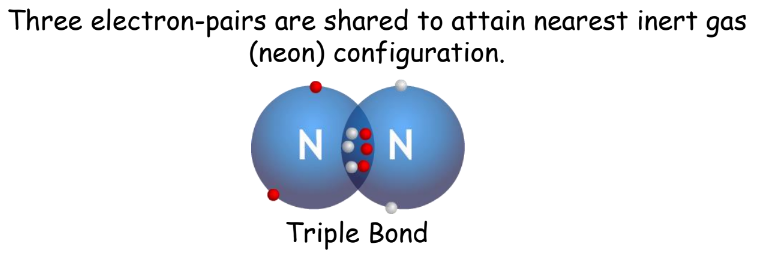
Carbon: A Unique Element
Carbon’s uniqueness stems from:
- Catenation: The ability to form long chains by bonding with other carbon atoms.
- Stable Covalent Bonds: Carbon forms stable covalent bonds with many elements.
- Multiple Bonds: Carbon can form single, double, or triple bonds.
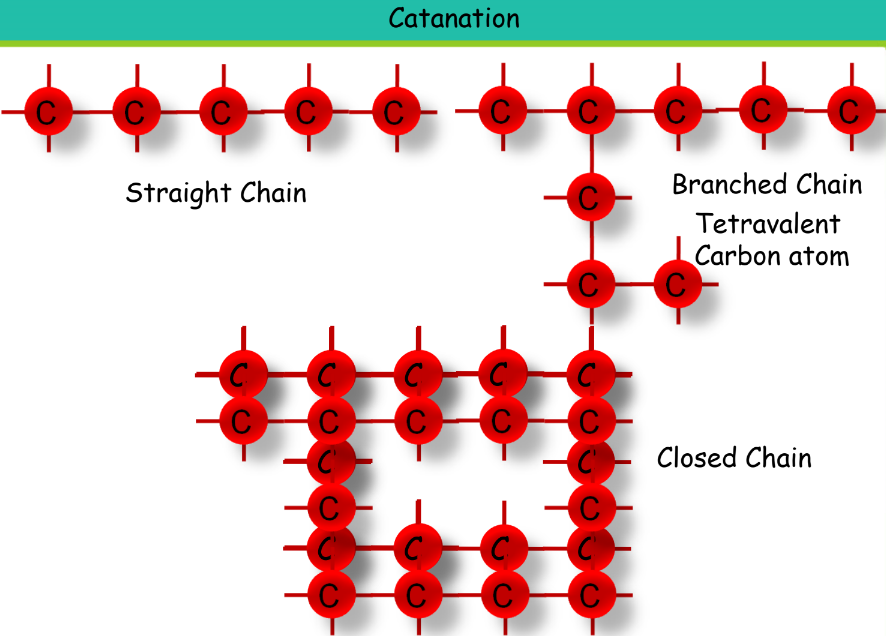
Allotropes of Carbon
Carbon exists in various allotropic forms with different physical properties but identical chemical properties.
Crystalline Forms
- Diamond: Hardest naturally occurring mineral, a bad conductor of electricity.
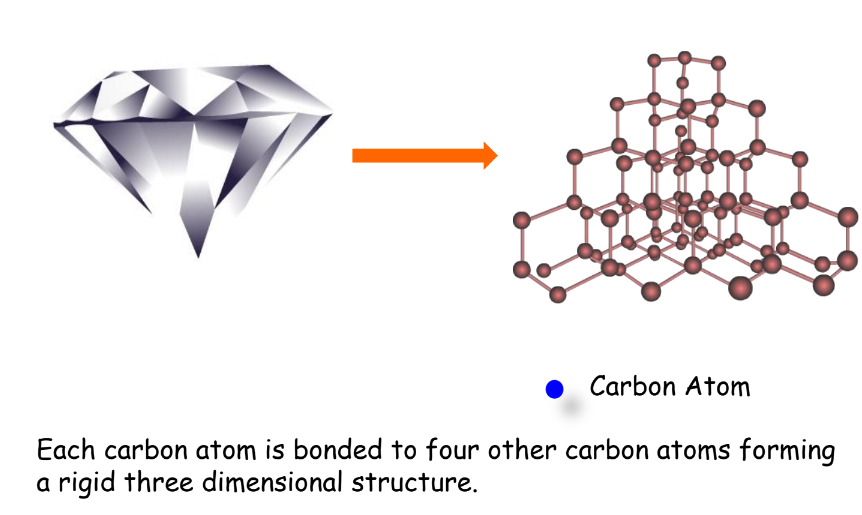
- Graphite: Smooth, slippery, and a good conductor of heat and electricity.
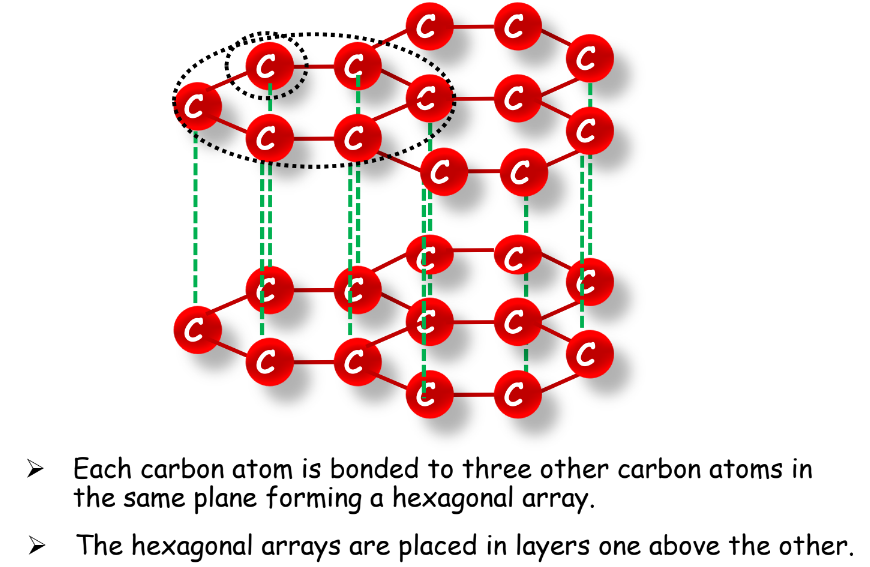
- Fullerene: Spherical or cylindrical arrangements of carbon atoms, such as buckyballs and nanotubes.

Amorphous Forms
- Coal
- Coke
- Charcoal
Hydrocarbons
Types of Hydrocarbons
Hydrocarbons are organic compounds consisting solely of carbon and hydrogen. They are classified into:
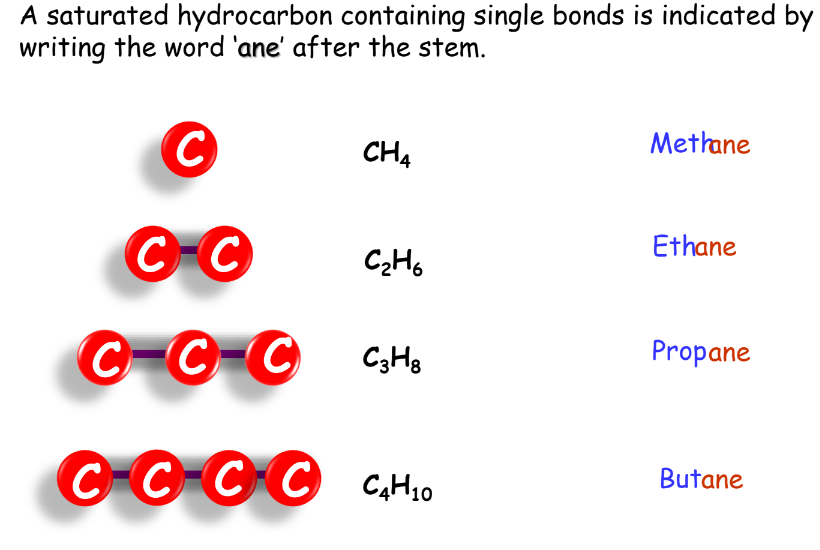
- Saturated Hydrocarbons (Alkanes): Contain only single bonds (C-C).
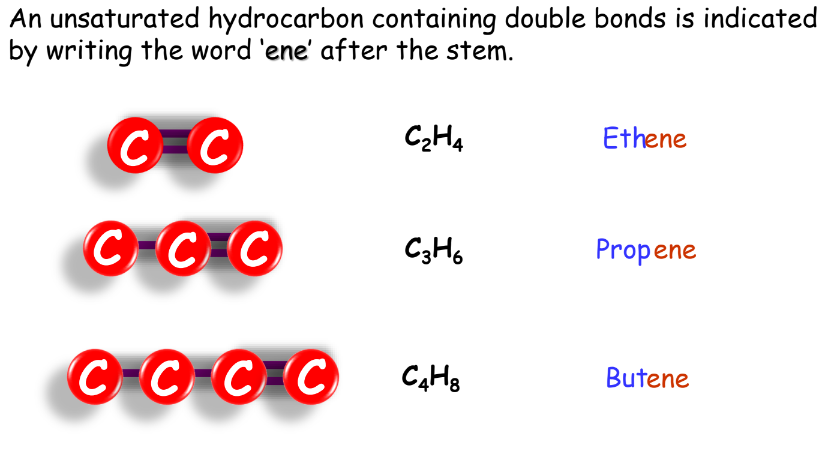
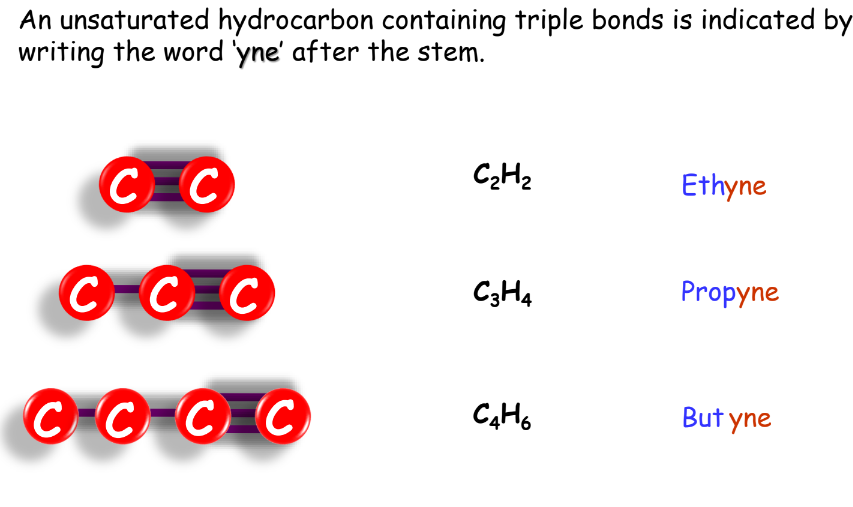
- Unsaturated Hydrocarbons: Contain double (Alkenes) or triple (Alkynes) bonds.
Structure of Hydrocarbons
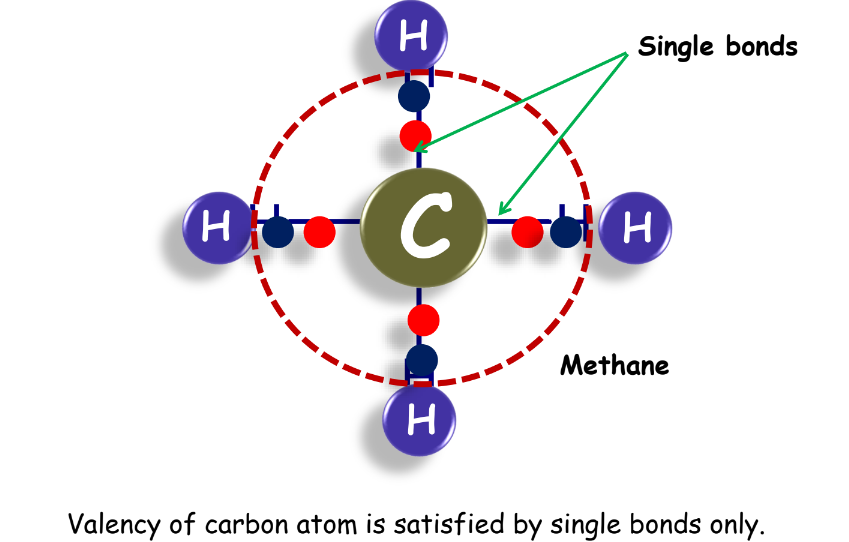
- Methane: Simplest hydrocarbon with a single bond.
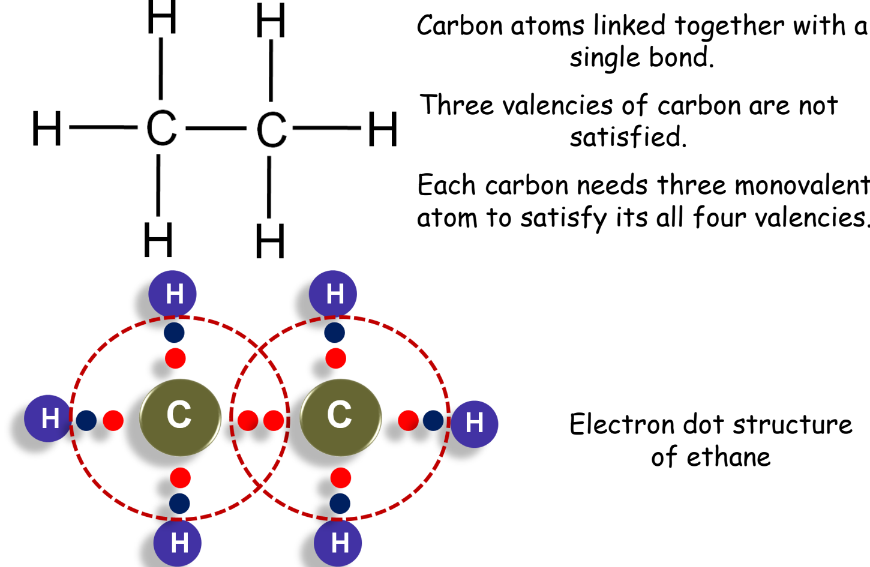
- Ethane: Two carbon atoms linked by a single bond.
- Propane: Three carbon atoms, forming a chain.
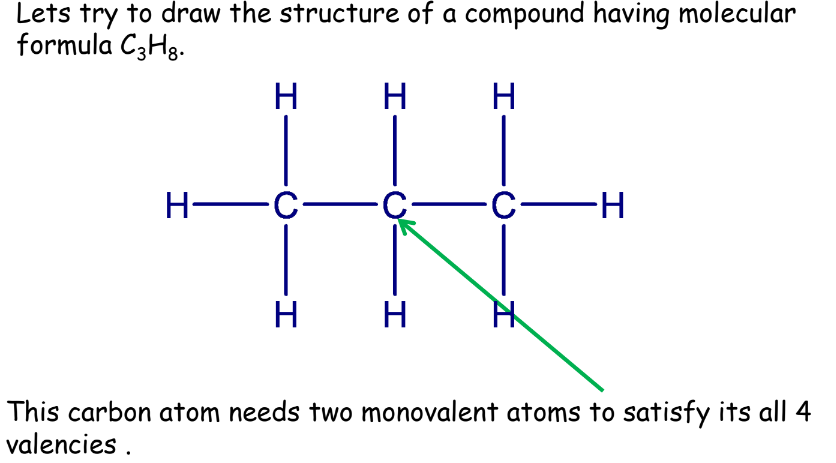
Therefore, for saturated hydrocarbons we can say that:

Chains, Branches, and Rings
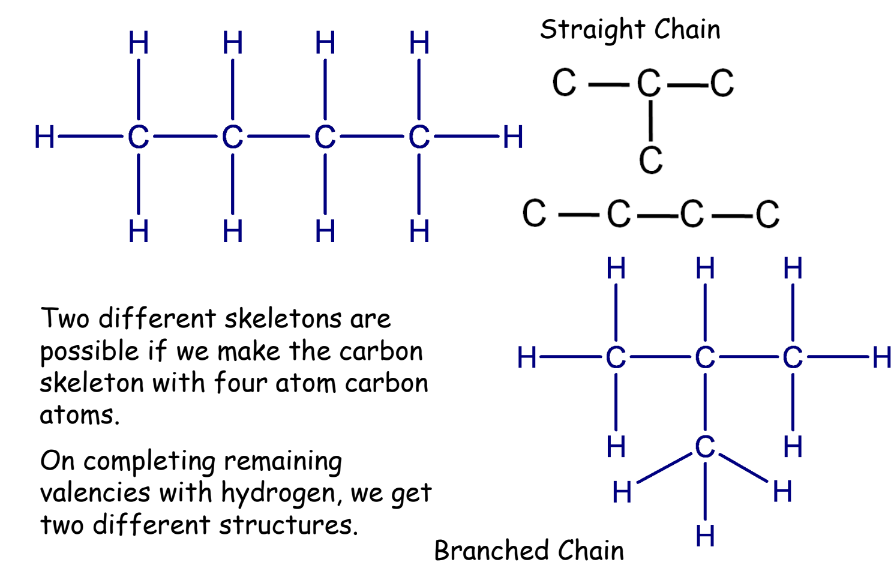
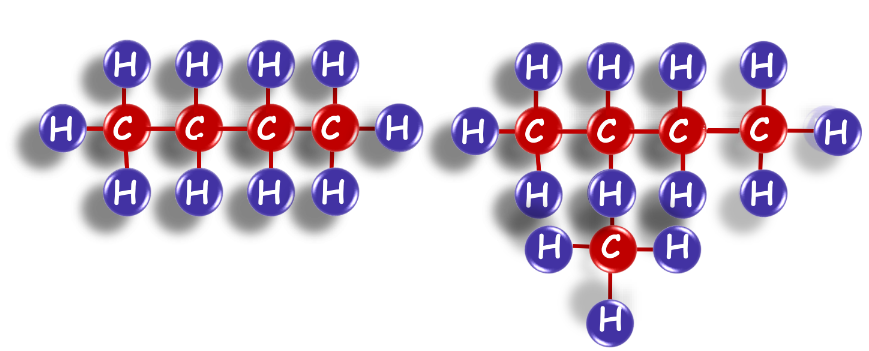
We can see that both these structures have the same formula. Such compounds are called structural isomers.
Isomerism
Isomerism refers to compounds having the same molecular formula but different structures, known as structural isomers. Isomerism is common in hydrocarbons with four or more carbon atoms.
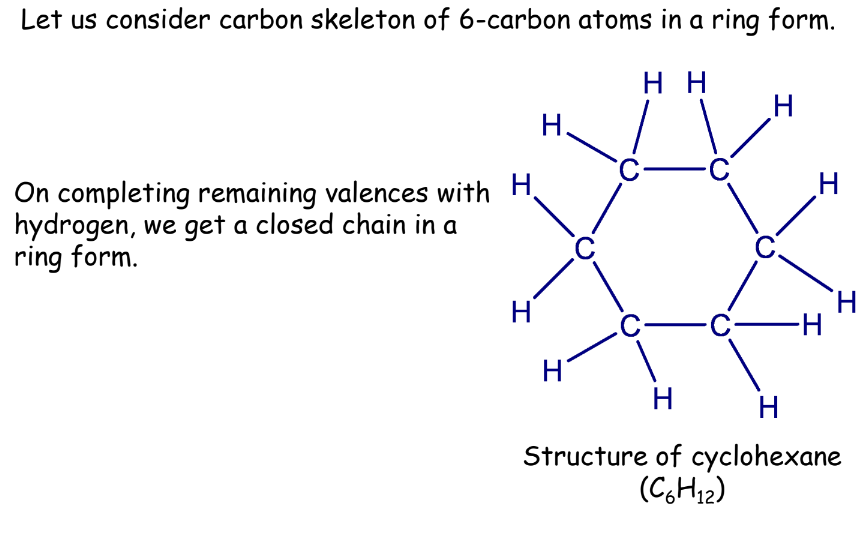
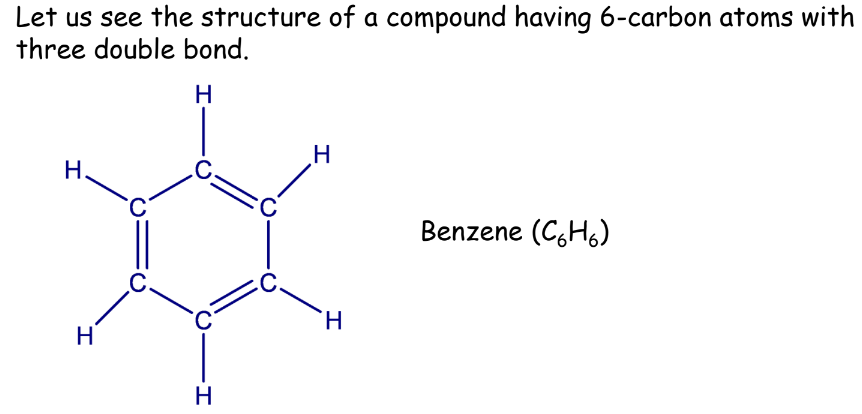
Unsaturated Hydrocarbons
- Alkenes: Contain at least one carbon-carbon double bond.
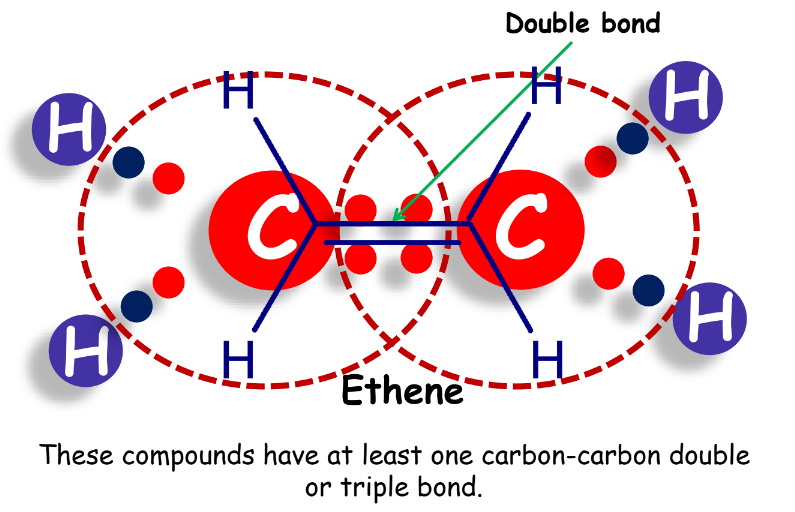
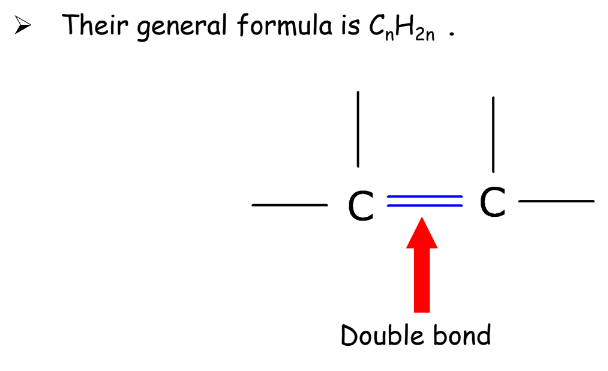
- Alkynes: Contain at least one carbon-carbon triple bond.
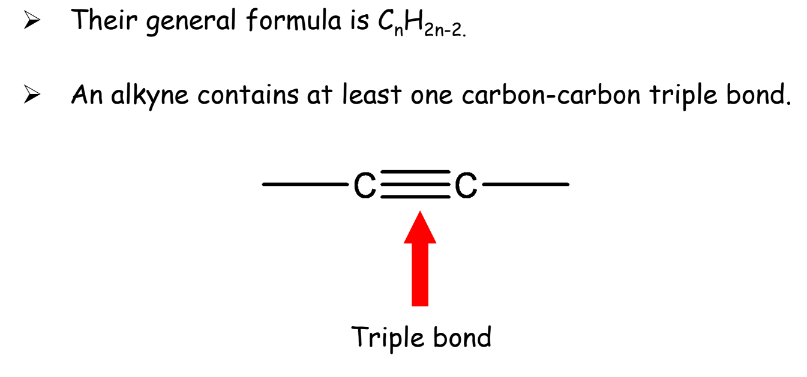
Rules:
- If the number of hydrogen atoms is 2 more than double the number of carbon atoms, then it will be an alkane.
- If the number of hydrogen atoms is exactly equal to double the number of carbon atoms, then it will be an alkene.
- If the number of hydrogen atoms is 2 less than double the number of carbon atoms, then it will be an alkyne.
Nomenclature of Organic Compounds
There are two systems employed in the naming of the organic compounds.
- Trivial system
- IUPAC system
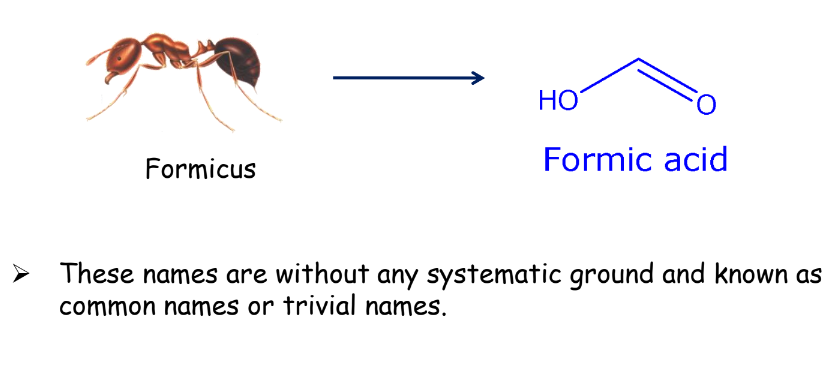
Trivial System: In the beginning, the organic compounds were named after the source from which they were extracted or obtained.
Formic acid derived its name from the Greek word formicus (red ants) since the acid could be obtained from them.
IUPAC System: To rationalize the system of naming organic compounds, IUPAC (International Union of Pure and Applied Chemistry) came into being in 1892 in Geneva. This system adopted certain uniform rules for naming the compounds.
Naming of Alkanes, Alkenes, and Alkynes
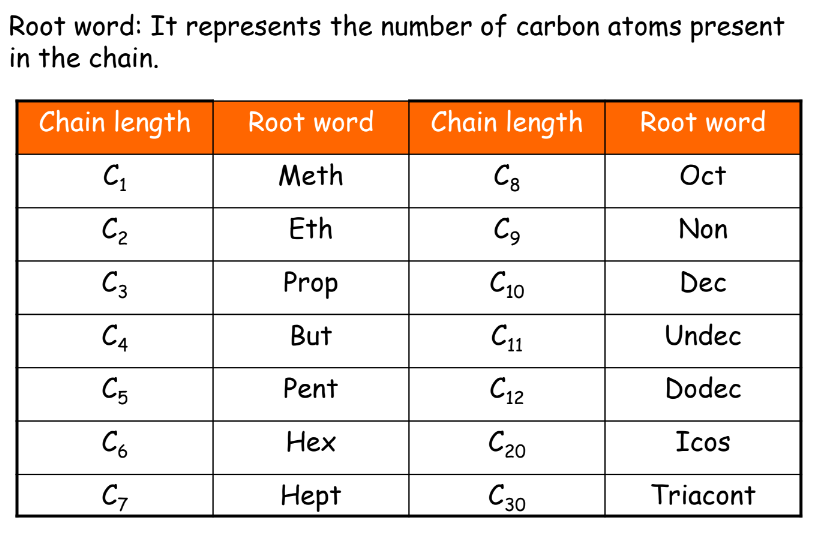
- Alkanes: Named with the suffix ‘-ane’.
- Alkenes: Named with the suffix ‘-ene’.
- Alkynes: Named with the suffix ‘-one’.
Homologous Series
- It is a group of organic compounds having similar structures and similar chemical properties in which the successive compounds differ by CH2 (14 amu) group.
- All the alkanes have similar structures with single covalent bonds and show similar chemical properties, so they can be grouped in the form of a homologous series.
- All the members can be represented by the same general formula.
- All the compounds of a homologous series show similar chemical properties.
- The members of a homologous series show a gradual change in their physical properties with an increase in molecular mass.
Functional Groups
A functional group is an atom or group of atoms that determine the chemical properties of a compound.
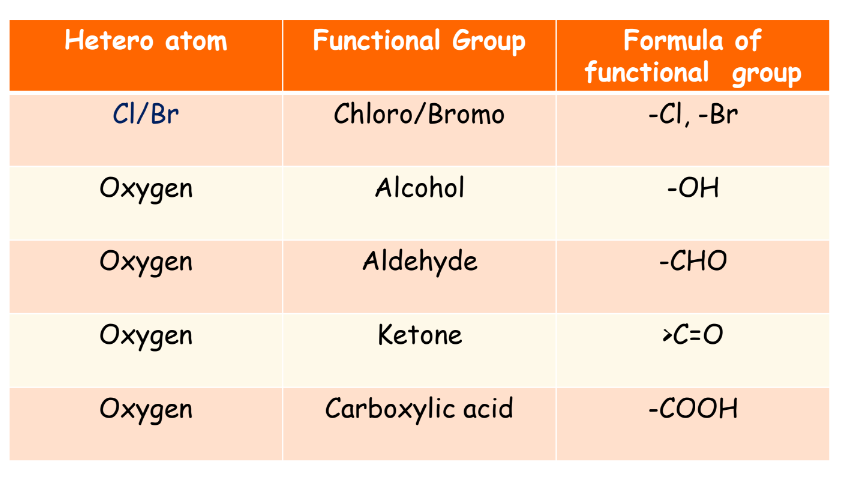
Functional Group
Prefix/Suffix
Example
Halogen
Prefix: Chloro, Bromo, etc.
Chloroethane
Alcohol
Suffix: -ol
Ethanol
Aldehyde
Suffix: -al
Ethanal
Ketone
Suffix: -one
Propanone
Carboxylic Acid
Suffix: -oic acid
Ethanoic acid
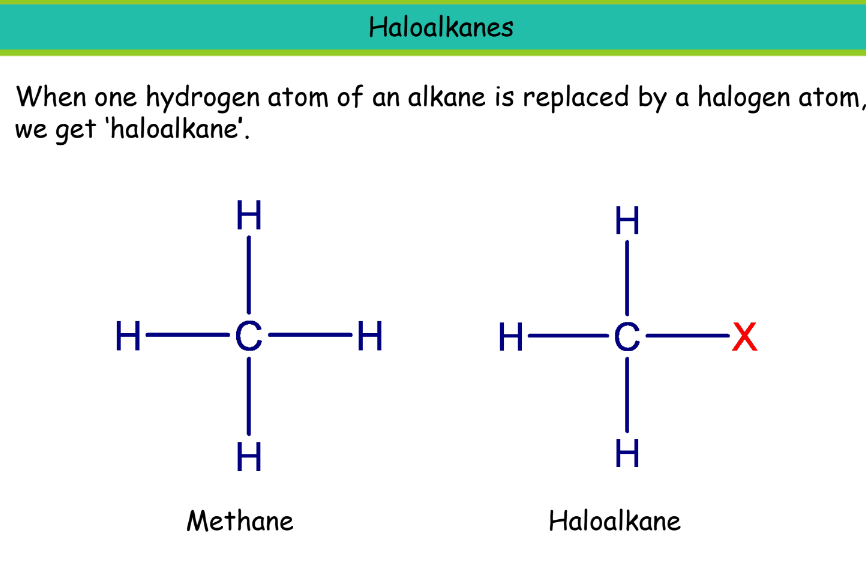
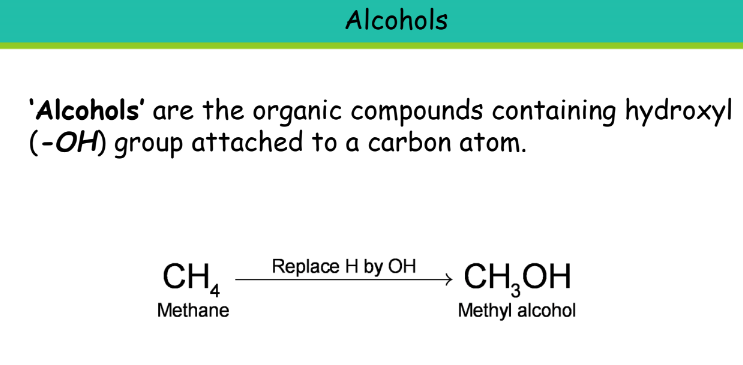
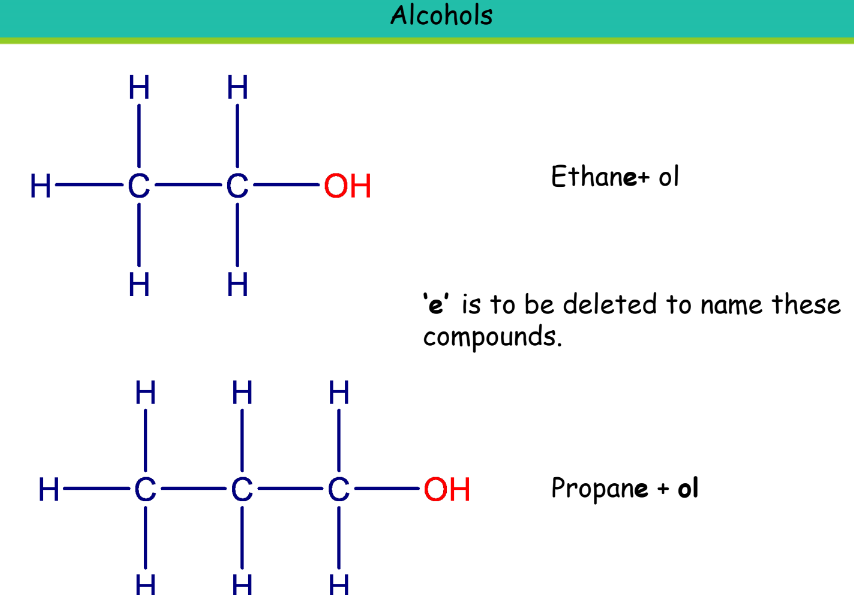
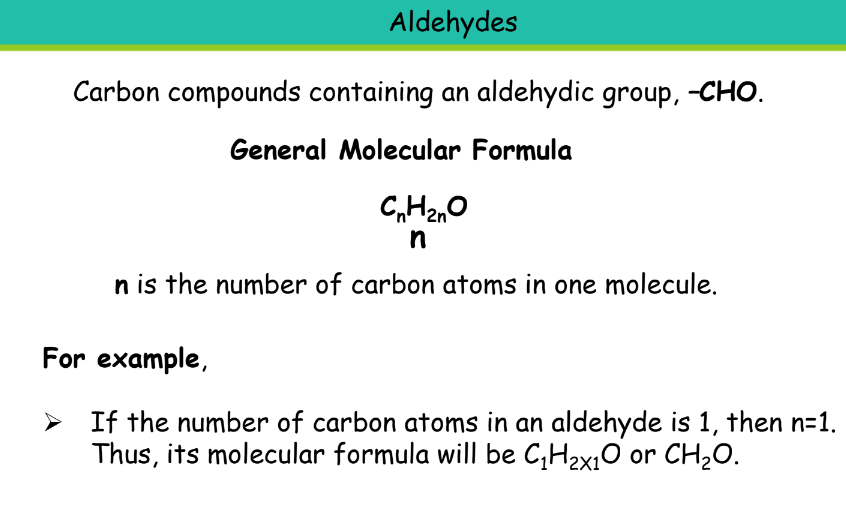
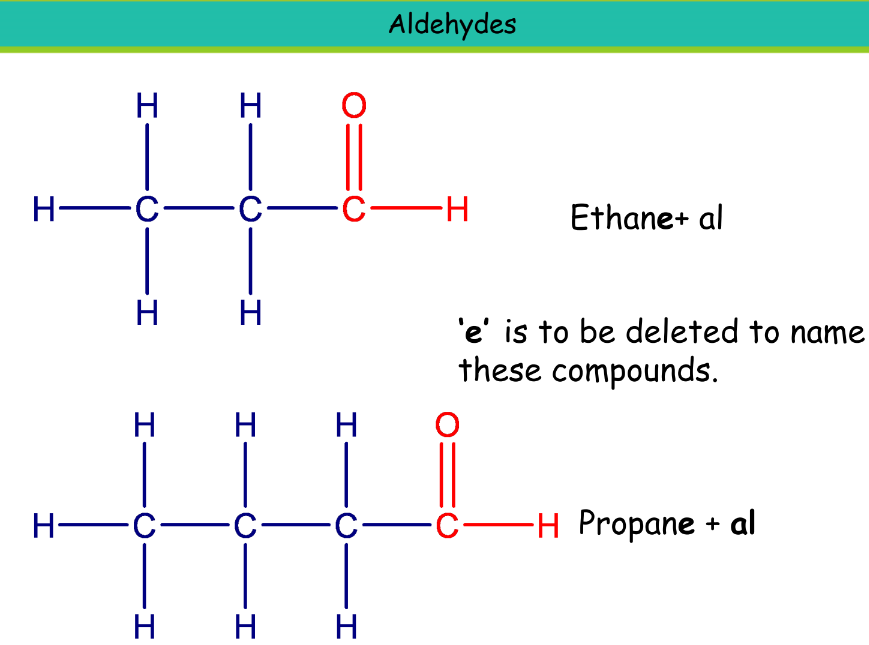
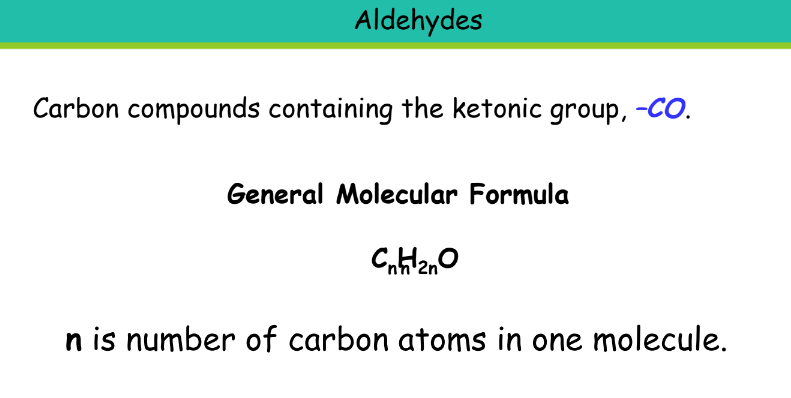
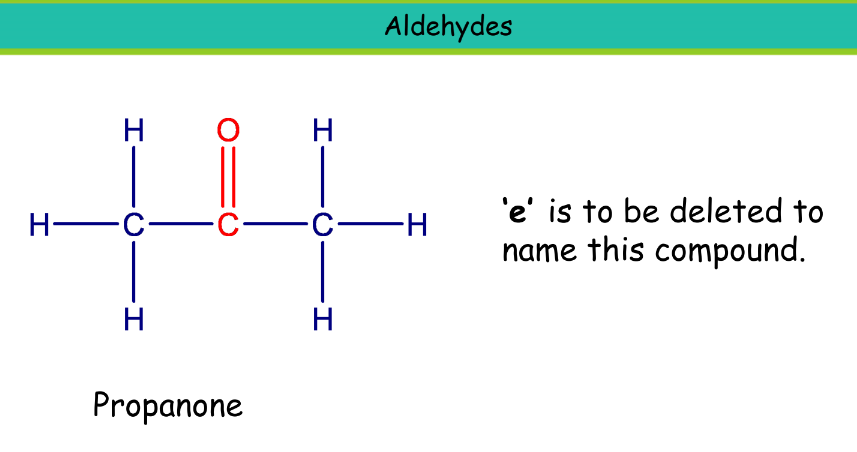
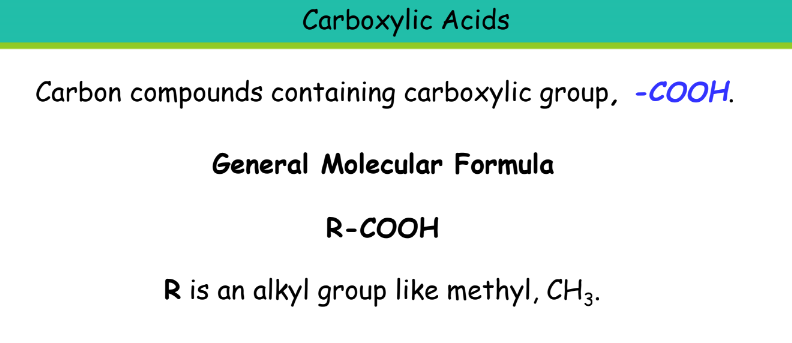
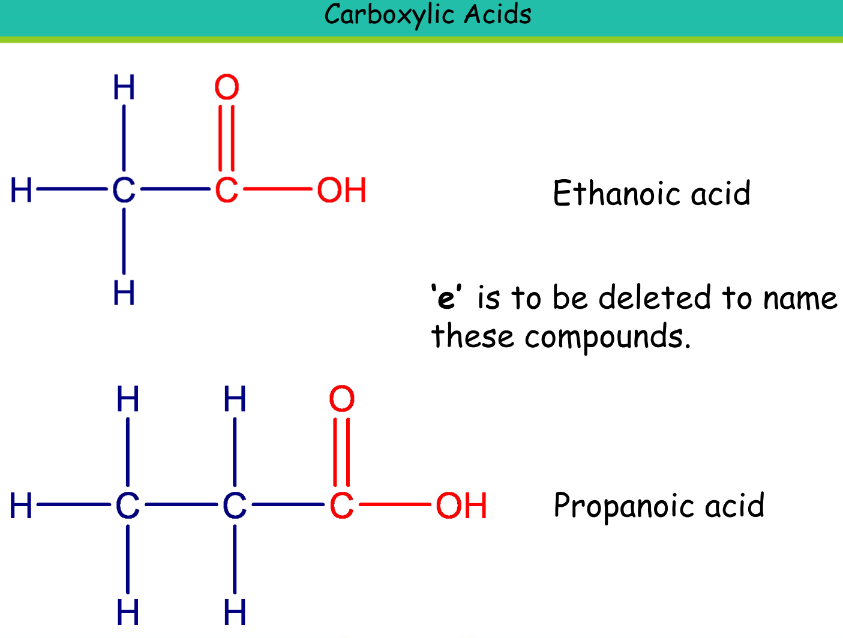
Chemical Properties of Carbon Compounds
Combustion Reactions
Combustion is the process of burning carbon compounds in the air to produce carbon dioxide, water, and heat. For example, methane burns in the air to produce carbon dioxide and water, releasing a significant amount of heat.
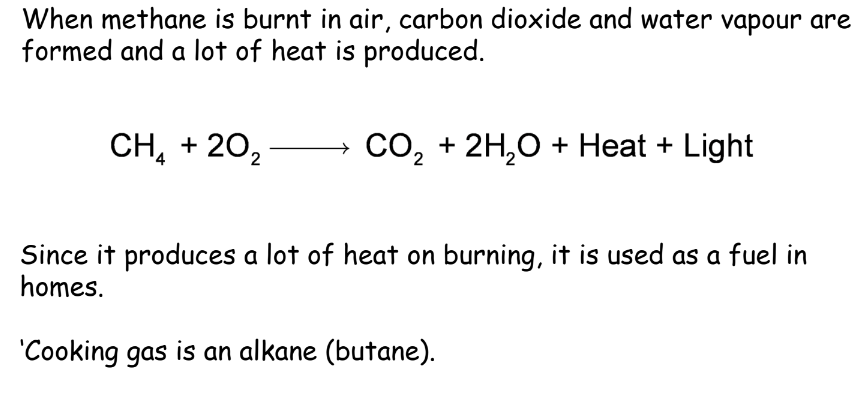
- The saturated hydrocarbons generally burn in the air with a blue, non-sooty flame.
Reason: The percentage of carbon in saturated hydrocarbons is comparatively low which gets oxidized completely by the oxygen present in the air.
- If the supply of air for burning is reduced, then incomplete combustion of even saturated hydrocarbons will take place and will burn, producing a sooty flame (giving a lot of black smoke).
Combustion of Unsaturated Hydrocarbons
- The unsaturated hydrocarbons burn in the air with a yellow, sooty flame (producing black smoke). For example, Ethene and ethyne burn in the air with a sooty flame.
Oxidation
Oxidation involves the addition of oxygen to carbon compounds. Oxidizing agents are substances that bring about oxidation.
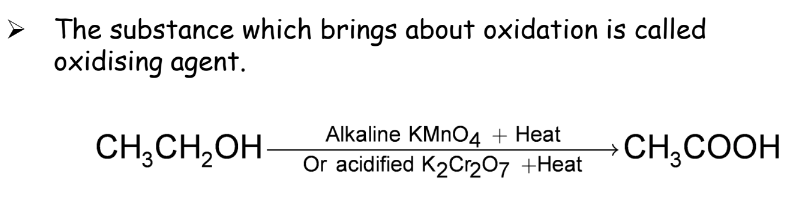
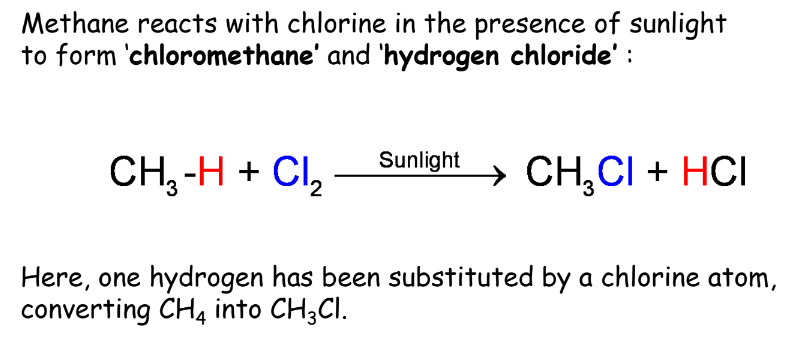
Substitution Reactions
Substitution reactions involve the replacement of one or more hydrogen atoms in a hydrocarbon with another atom or group of atoms.
Addition Reactions
Addition reactions occur when an unsaturated hydrocarbon reacts with another substance to form a single product. Hydrogenation of oils is an example where unsaturated fats are converted into saturated fats.
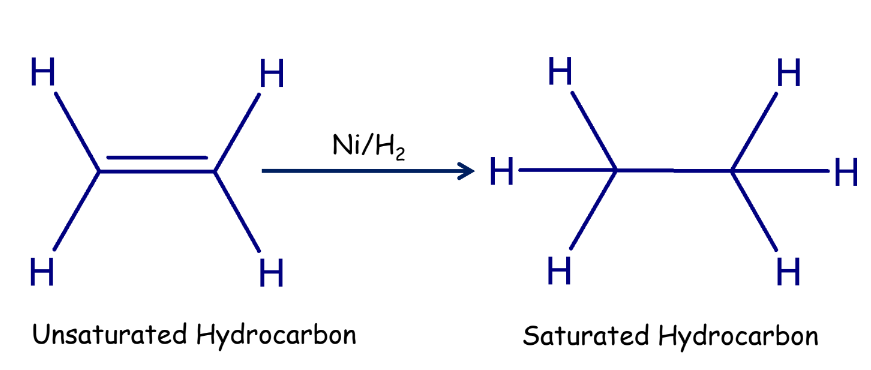
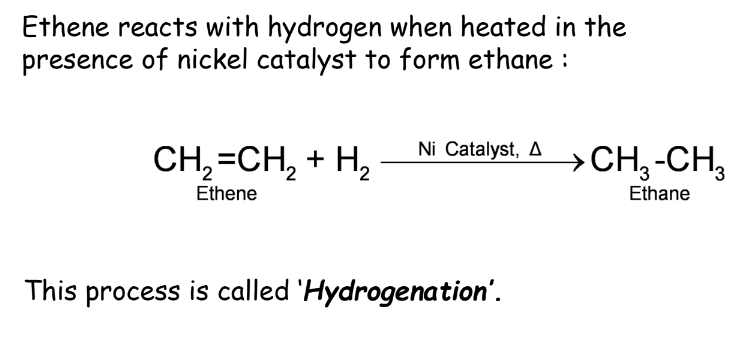
Hydrogenation of Oils
- Vegetable oils are unsaturated fats that have double bonds between some of their carbon atoms.
- When vegetable oil is heated with hydrogen in the presence of a nickel catalyst, then a saturated fat called ‘VEGETABLE GHEE’ is formed.
Important Carbon Compounds
As per the chapter carbon and its compounds, there are various important carbon compounds around us. these involve-
Ethanol

- Properties: Colorless, volatile, flammable liquid, used as a solvent and in alcoholic beverages.
- Chemical Properties

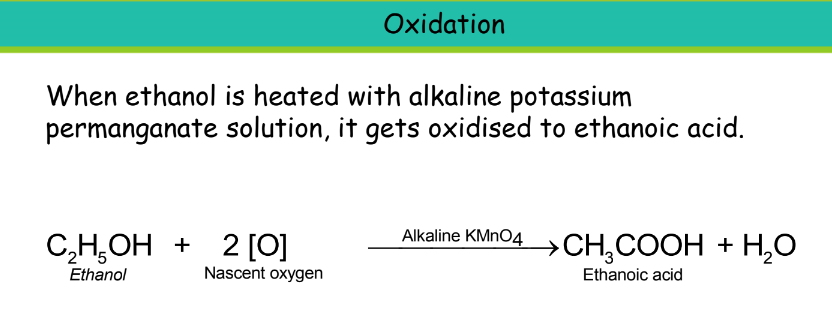
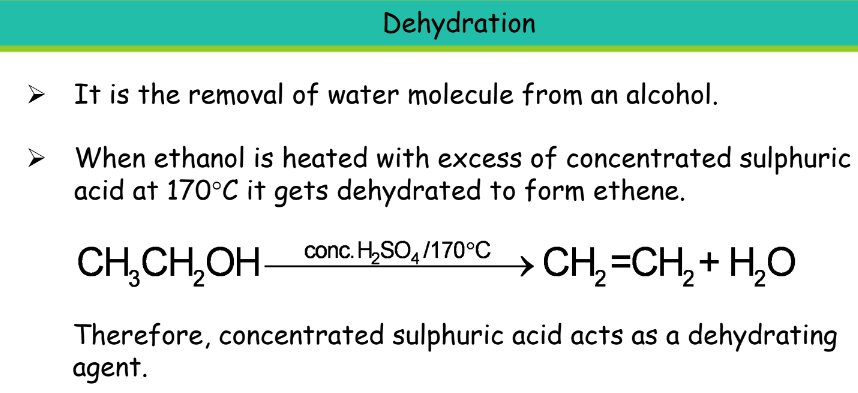
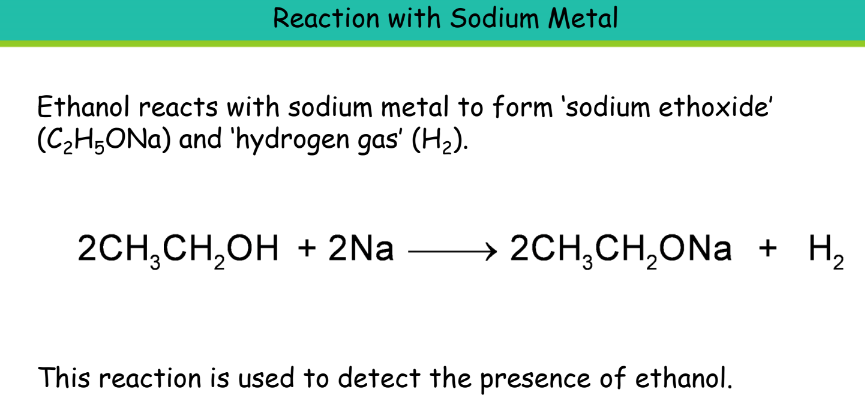
Uses: It is used in the manufacture of paints, medicines, perfumes, dyes, soaps, and synthetic rubber. It is used as a fuel in cars along with petrol. Also, used in alcoholic drinks like wine, whisky beer, and other liquors. Vinegar is an aqueous solution of ethanoic acid.
Acetic Acid/ Ethanoic Acid
The chapter carbon and its compounds also covers the topic of acetic acid and ethanoic acid. This involves –
- Properties: Colorless liquid with a pungent smell, used in vinegar and as a chemical reagent. It has a Boiling point = 118C and Melting point = 7C. Therefore, it freezes in winter in cold climates and hence, is known as glacial acetic acid. Miscible with water in all proportions and weak acid as compared to mineral acids.
- Chemical Properties:
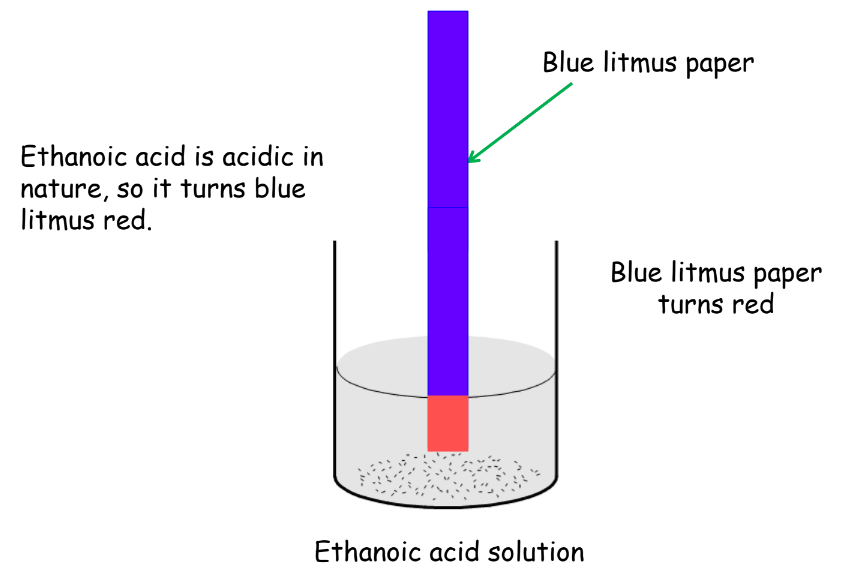
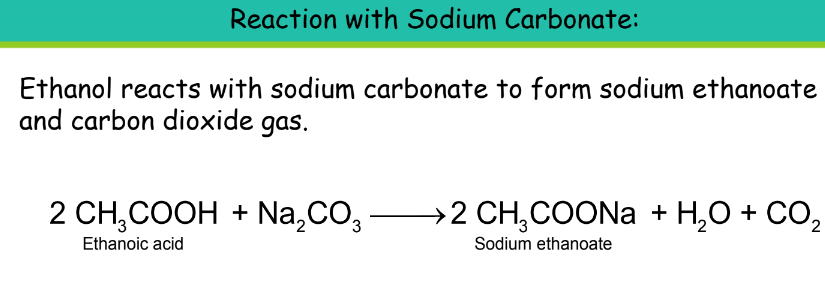
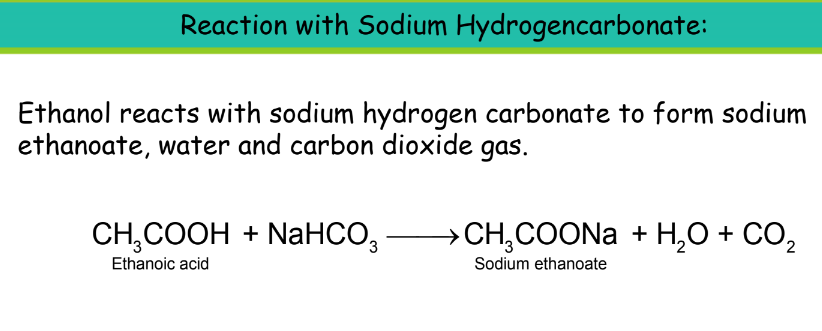
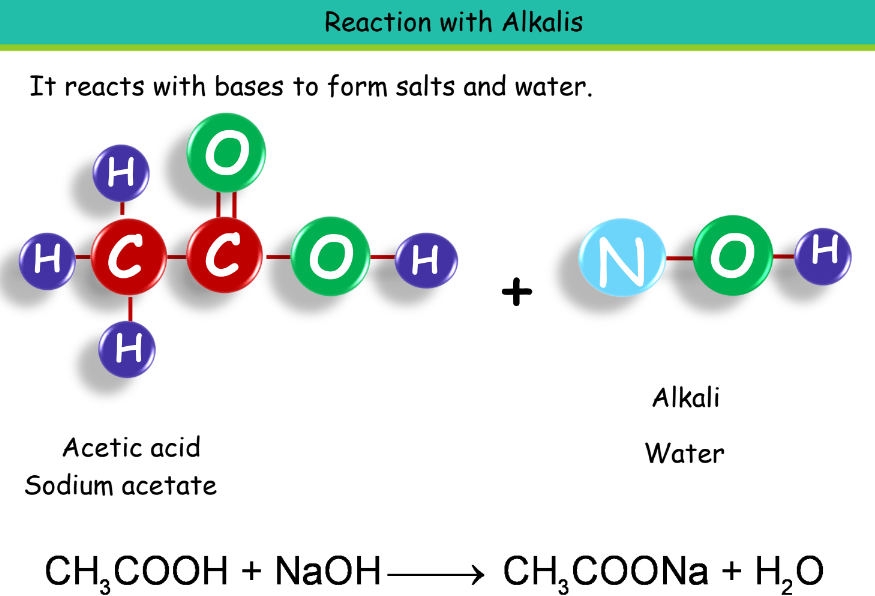
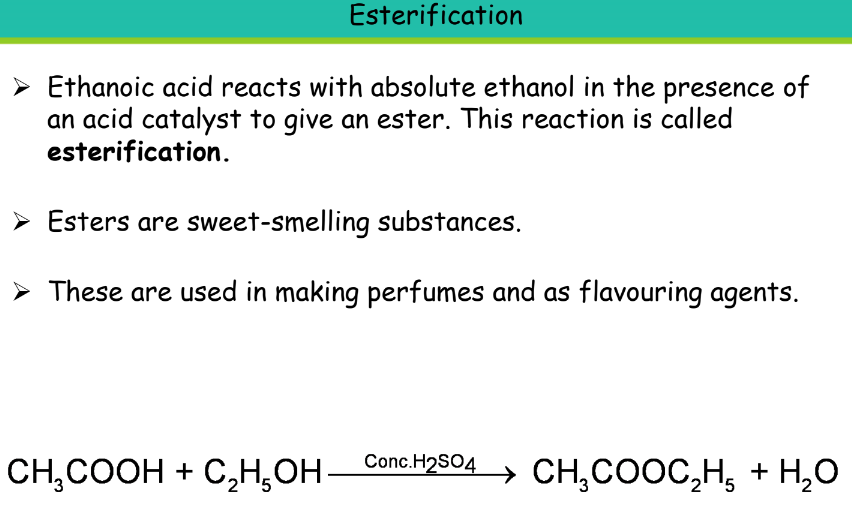
Uses of Ethanoic Acid:
- It is used as a food preservative while storing pickles, for making cellulose acetate which is an important artificial fibre. Also used in the preparation of dyes, and plastics.
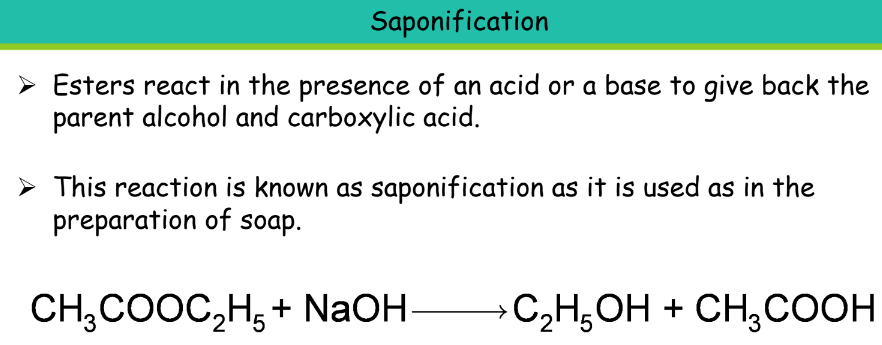
Soaps and Detergents
Soaps
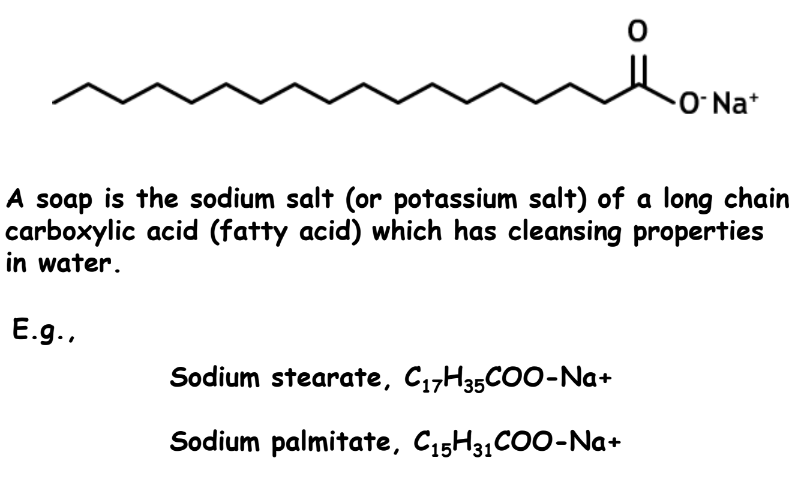
Preparation of soaps:
Soap is made by heating animal fat or vegetable oil with a concentrated sodium hydroxide solution.

Cleansing Action of Soaps & Detergents
Soaps and detergents have two parts:
- A non-polar part consists of a long chain hydrocarbon part called a tail. It is hydrophobic.
- An ionic part consisting of carboxylate ions is called the polar head. Itis hydrophilic.
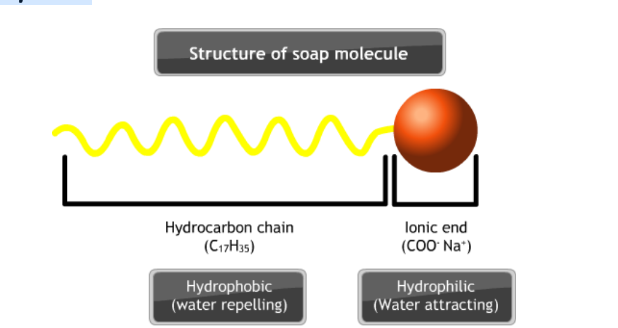
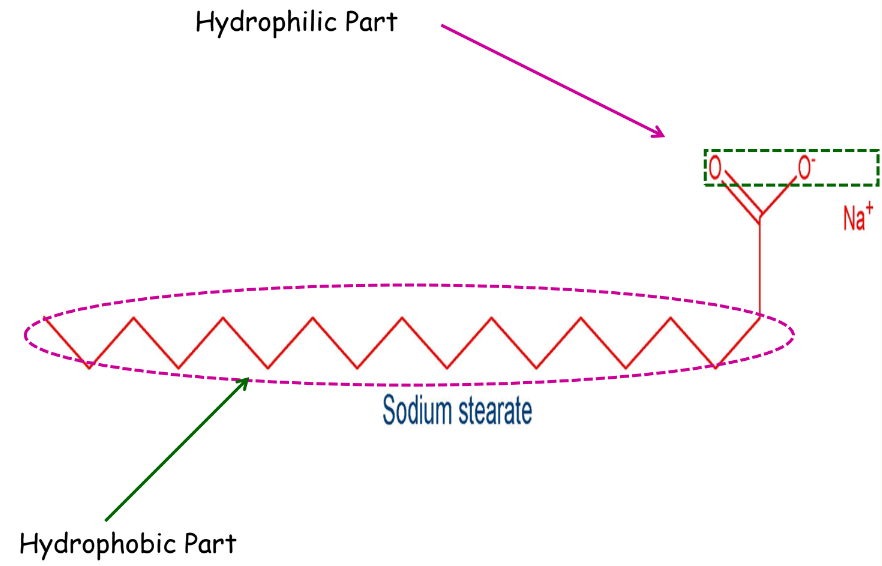
When soap is dissolved in water, it forms a colloidal suspension in water. The soap molecules are arranged radially with hydrocarbon ends directed towards the center and ionic ends directed outwards.
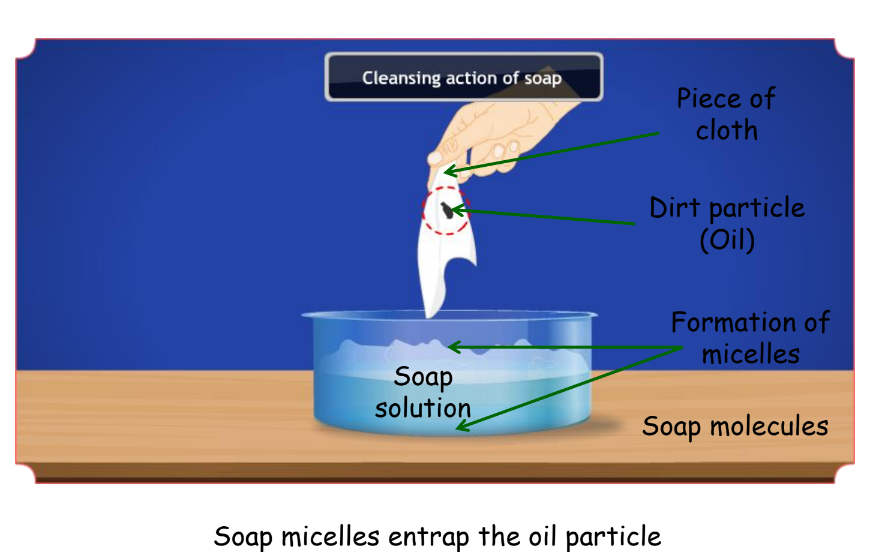
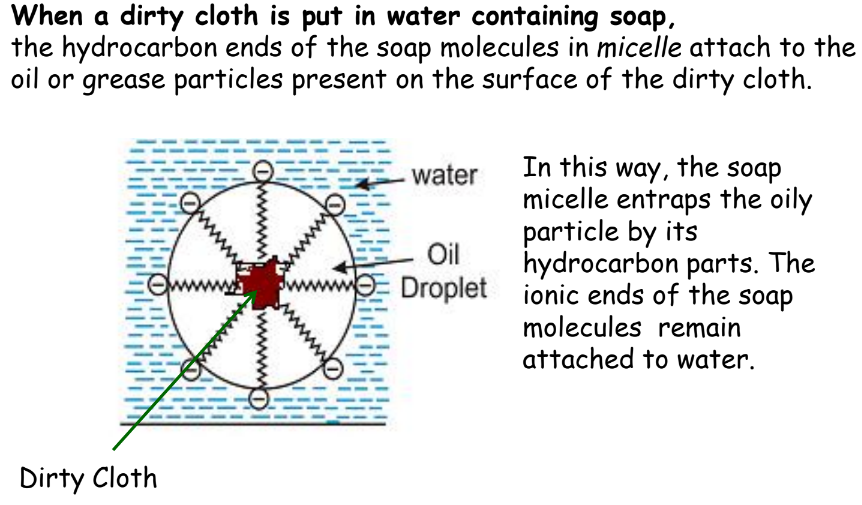
- When the dirty cloth is agitated in soap solution, the oily and greasy particles present on its surface are entrapped by soap micelles.
- They get dispersed in water due to which the soap water becomes dirty and the cloth is cleaned.
Detergents
The chapter on carbon and its compounds gives a clear understanding of Detergents. These are “soap-less soaps”. They have soap-like cleansing properties but they
don’t contain chemicals like sodium stearate etc. are better cleansing agents than soaps. They do not form insoluble salts with hard water.
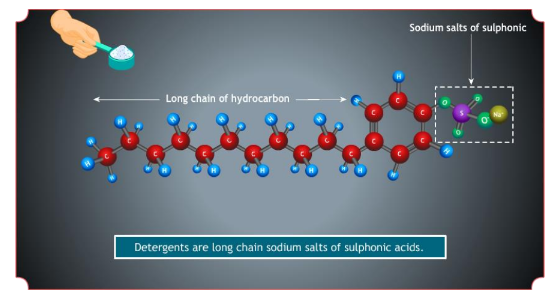
Example:
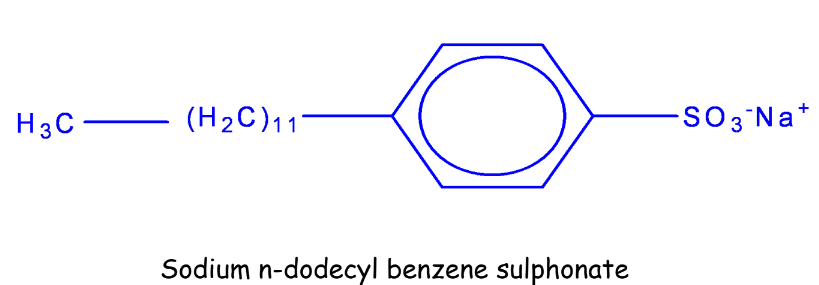
Uses of detergents:
- They can be used even with hard water for washing purposes.
- They have a stronger cleansing action than soaps.
- They are more soluble in water than soaps.
If you liked this summary and want to read a similar post on Class 10 Science Chapter 13 – Our Environment, click here.
Conclusion
In conclusion, CBSE Class 10th Science Chapter 4 – Carbon and Its Compounds offers an in-depth understanding of one of the most versatile elements on Earth. From its ability to form countless compounds to its significant role in organic chemistry, carbon’s importance cannot be overstated. Whether through the formation of covalent bonds, isomerism, or its allotropic forms, the study of carbon opens the door to a deeper comprehension of the chemical world.
With iPrep Learning Super App, mastering CBSE Class 10th Science Chapter 4 – Carbon and Its Compounds becomes easier and more engaging. Our comprehensive resources, including videos, notes, and practice questions, ensure that you are well-prepared for both exams and real-world applications. So dive into the world of carbon and its compounds, and let iPrep guide you toward academic excellence.
Practice questions on Chapter 4 - Carbon And Its Compounds
Get your free Chapter 4 - Carbon And Its Compounds practice quiz of 20+ questions & detailed solutions
Practice Now








