Combustion And Flame – Complete Guide For Class 8 Science Chapter 4
Welcome to iPrep, your Learning Super App! Our learning resources for the chapter, Combustion, and Flame in Science Class 8th Chapter 4 are designed to ensure that you grasp this concept with clarity and perfection. Whether you’re studying for an upcoming exam or strengthening your concepts, our engaging animated videos, practice questions and notes offer you the best of integrated learning with interesting explanations and examples.
The concept of Combustion and flame in Class 8 Science introduces students to a comprehensive understanding of chemical reactions that produce energy. These processes are not only fascinating but also play a crucial role in everyday life, from cooking to powering vehicles. In this topic, we’ll explore the intricacies of combustion, the characteristics of flames, and the impacts of these reactions.
What is Combustion?
Combustion is a chemical process in which a substance reacts with oxygen in the air to produce heat and light. This process involves two primary types of substances:
- Combustible Substances: These materials easily burn or catch fire. Examples include wood, coal, paper, and cloth.

- Non-combustible Substances: These materials do not catch fire easily. Examples include glass, stone, and iron.

Ignition Temperature
The ignition temperature is the minimum temperature at which combustion can occur, as it is required for a substance to catch fire and begin burning. Combustible substances cannot ignite until their temperature reaches this point. For example, white phosphorus has a low ignition temperature of about 35°C and can ignite at room temperature, so it is stored in water to prevent accidental fires.
Inflammable Substances
Inflammable substances have very low ignition temperatures and can easily undergo combustion when exposed to a flame. Common examples include petrol and Liquified Petroleum Gas (LPG).
Essential Requirements for Combustion
For combustion to occur, three essential elements must be present:
- Fuel: The substance that burns. Fuels can be solid (e.g., wood, coal), liquid (e.g., kerosene, diesel), or gas (e.g., methane, hydrogen).
- Air: Oxygen from the air is necessary for combustion. If the oxygen supply is cut off, combustion will stop. A simple experiment demonstrates this: place a glass chimney over a lit candle on wooden blocks, then cover the chimney with a glass plate. Removing the blocks cuts off the air supply and extinguishes the flame.
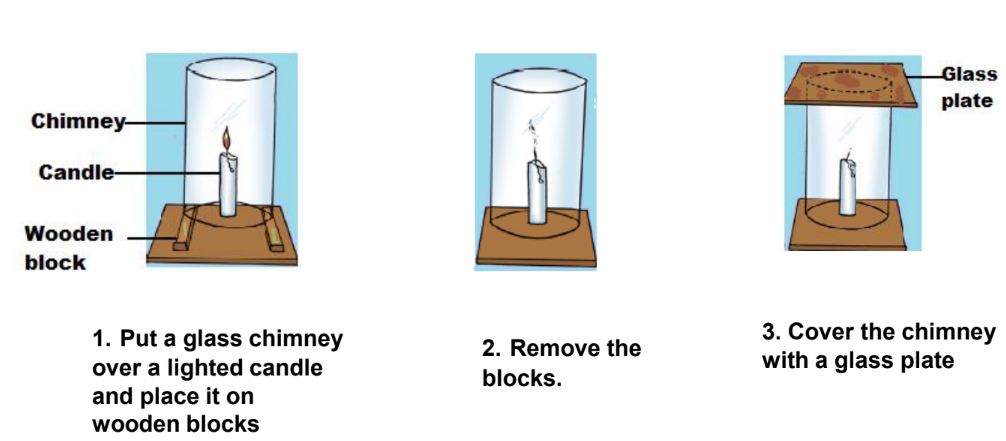
- Heat: Heat is needed to raise the fuel’s temperature beyond its ignition point. For example, heating paper increases its temperature until it reaches its ignition temperature and catches fire.
Controlling Fire
To control or extinguish a fire, one or more of the essential requirements for combustion must be removed. Fire extinguishers work by either stopping the air supply, lowering the fuel’s temperature, or both. Water and carbon dioxide are common fire extinguishers.
Types of Combustion
- Rapid Combustion: This type of combustion occurs when a material burns quickly, producing heat and light. Phosphorus, for example, burns rapidly in the air at room temperature.
- Spontaneous Combustion: This type of combustion occurs when a material suddenly ignites without any apparent cause. Spontaneous forest fires can happen due to the sun’s heat.
- Explosion: This is a sudden reaction that produces heat, light, sound, and gas. A typical example is the ignition of fireworks.

Understanding Flames
A flame is the visible part of a fire, which emits light and heat due to the vaporization of a burning substance. In a kerosene lamp, for instance, the kerosene oil rises through the wick, vaporizes, and forms a flame.

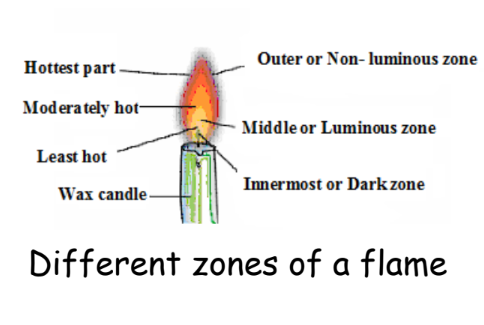
Different Zones of a Flame
A flame has three distinct zones, each with unique characteristics:
- Outer or Non-luminous Zone: The hottest part of the flame, where complete combustion occurs. This zone appears blue due to the high temperature.
- Middle or Luminous Zone: This moderately hot zone is where incomplete combustion occurs, resulting in unburnt carbon particles. This zone is usually bright yellow or orange.
- Innermost or Dark Zone: The least hot part of the flame, appearing black due to unburnt vapors of the substance, such as wax.
Characteristics of an Ideal Fuel
An ideal fuel is one that supports efficient combustion and should have the following characteristics:
- Availability and Cost: It should be readily available and inexpensive.
- Calorific Value: It should have a high calorific value, meaning it releases a significant amount of heat energy upon burning.
- Safety: It should burn without emitting harmful gases.
- Ignition Temperature: It should have an appropriate ignition temperature.
- Residue: It should not leave undesirable residues after burning.
Fuel Efficiency
Fuel efficiency is measured in terms of calorific value, defined as the amount of heat energy released during the complete combustion of 1 kg of fuel. Its unit is kilojoules per kilogram (kJ/kg).
Harmful Products of Combustion
Combustion can produce several harmful by-products, including:
- Unburnt Carbon Particles: These are pollutants produced by burning carbon-based fuels like coal and wood, causing respiratory diseases such as asthma and bronchitis.
- Carbon Monoxide: A poisonous gas resulting from incomplete combustion. It is essential to avoid sleeping in closed rooms with burning wood or coal fires due to the risk of carbon monoxide poisoning.

- Oxides of Sulphur and Nitrogen: These gases, produced by burning fossil fuels, lead to acid rain. Acid rain can damage crops, buildings, and soil.
- Carbon Dioxide: Elevated levels of carbon dioxide in the atmosphere contribute to global warming, increasing Earth’s temperature and causing glaciers to melt, leading to floods in coastal areas.
This comprehensive guide on coal and petroleum highlights how combustion and flame is crucial for managing energy resources efficiently and minimizing environmental impact. By learning about these processes, we can make informed decisions about fuel use and fire safety, contributing to a more sustainable future.
Practice questions on Chapter 4 - Combustion And Flame
Get your free Chapter 4 - Combustion And Flame practice quiz of 20+ questions & detailed solutions
Practice Now








