Thermodynamics – Complete Guide For Class 11 Physics Chapter 12
Welcome to iPrep, your Learning Super App. Our learning resources for Chapter 12, “Thermodynamics,” in Class 11 Physics are meticulously designed to ensure students gain a comprehensive understanding of this essential topic. These resources include detailed notes on fundamental concepts such as heat, work, and internal energy, along with thorough explanations of the Zeroth, First, and Second Laws of Thermodynamics. Students will also find in-depth coverage of various thermodynamic processes, including isothermal, adiabatic, and isochoric, and their applications in real-world scenarios. Additionally, the resources feature interactive diagrams and practical examples, such as heat engines and refrigerators, to illustrate complex principles effectively. Comprehensive exercises and problem sets are also provided to reinforce learning and ensure mastery of the material.
The concept of “Thermodynamics” in Class 11 Physics delves into the foundational principles of science by exploring the methods and standards used to quantify and describe physical phenomena. This chapter introduces students to the fundamental laws governing heat, energy, and work, emphasizing their practical implications. Students learn about the Zeroth, First, and Second Laws of Thermodynamics, and their applications in understanding energy transformations and system behaviors. The chapter also covers various thermodynamic processes, such as isothermal, adiabatic, and isochoric, providing a comprehensive understanding of how these processes impact different systems. Additionally, students explore the concepts of heat engines, refrigerators, and the Carnot cycle, which illustrate the real-world applications of thermodynamic principles.
What is Thermodynamics?
Thermodynamics studies the effects of work, heat, and energy on physical systems. Unlike other branches of physics that focus on molecular details, thermodynamics looks at large-scale effects on a system, such as pressure, temperature, and volume.
Thermal Equilibrium
A system is said to be in thermal equilibrium when its macroscopic variables—like pressure, temperature, and volume—remain constant over time. This concept is crucial in understanding the flow of heat and energy between systems.
Zeroth Law of Thermodynamics
The Zeroth Law of Thermodynamics states that if two systems (A and B) are in thermal equilibrium with a third system (C), then A and B are also in thermal equilibrium with each other. This law forms the basis for temperature measurement.
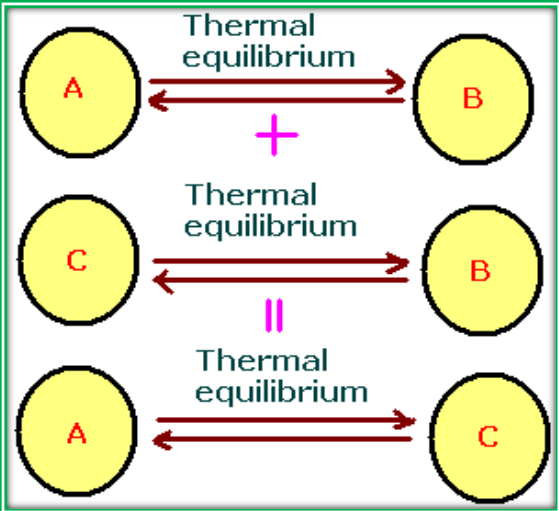
Systems Thermal Equilibrium A, C Yes B, C Yes A, B Yes
Heat and Internal Energy
- Heat: Heat is a form of energy that naturally flows from a body at a higher temperature to a body at a lower temperature.
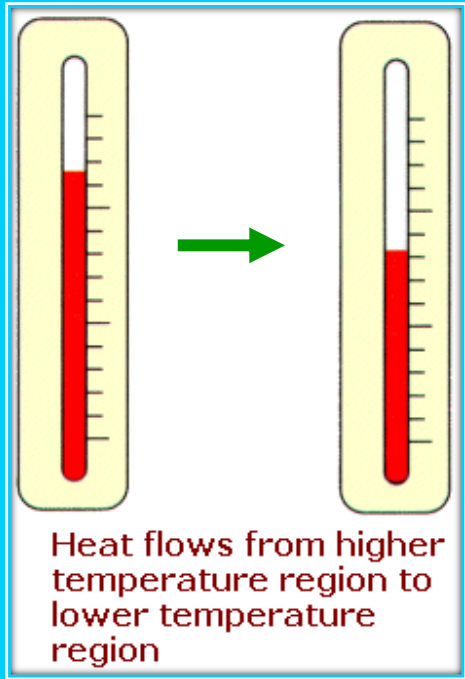
- Internal Energy: This is the total energy stored in a system due to molecular motion and configuration. It depends on the system’s state and not on how it arrived there.
Work and The First Law of Thermodynamics
Work in thermodynamics is the energy transferred without involving temperature differences. For example, pushing the piston in a gas-filled cylinder constitutes work.
The First Law of Thermodynamics is essentially the law of conservation of energy. It states: ΔQ=ΔU+ΔW.
- ΔQ is the heat supplied to the system,
- ΔU is the change in internal energy,
- ΔW is the work done by the system.
Specific Heat Capacity is the amount of heat required to raise the temperature of a unit mass of a substance by one degree Celsius (or Kelvin). It is a measure of a material’s ability to absorb heat and is expressed in joules per kilogram per degree Kelvin (J/kg·K). This property helps determine how different substances respond to heat energy changes. It is expressed as:
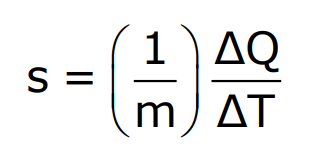
Thermodynamic State Variables: State variables are those macroscopic variables that determine the equilibrium state of a thermodynamic system. Examples: pressure, temperature, volume, and mass.
- The connection between state variables is called the equation of state.
- The equation of state for an ideal gas is represented as PV = μRT
- For a given amount of gas, only two variables are independent, that is either P and V or T and V.
Quasi-static Process: It is an infinitely slow process such that the system remains in thermal and mechanical equilibrium with the surroundings throughout.
Thermodynamic Processes
Thermodynamics involves various processes that help us understand energy transformations:
Isothermal Process
In an isothermal process, the system’s temperature remains constant. During an isothermal expansion of a gas, the work done is given by:
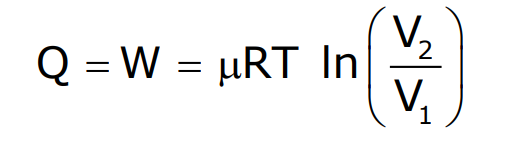
This process results in a curve called an isotherm when plotted on a pressure-volume graph.
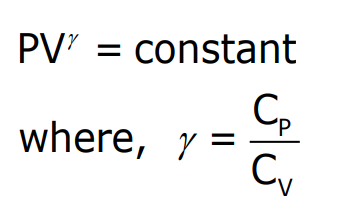
Adiabatic Process
In an adiabatic process, no heat is exchanged between the system and its surroundings. The temperature of the system changes due to internal factors. The relationship for an adiabatic process is:
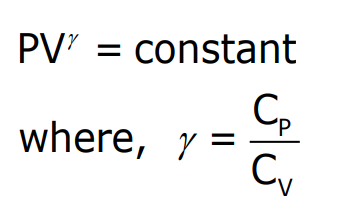
Isochoric Process
An isochoric process keeps the volume constant, meaning no work is done. The heat added only changes the internal energy and temperature.
Isobaric Process
In an isobaric process, the pressure remains constant. The work done in this process is: W=P(V2−V1)=μR(T2-T1)
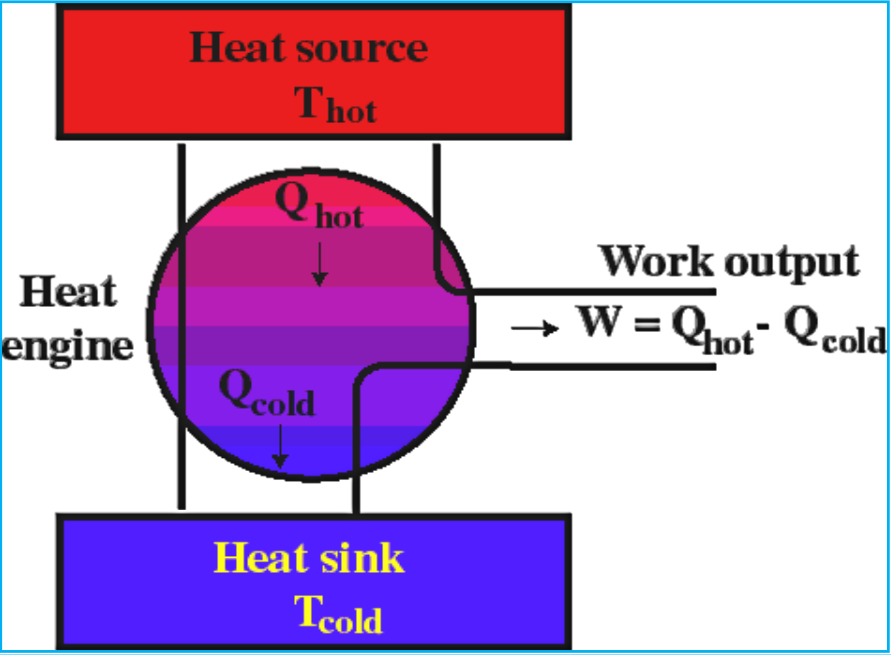
Heat Engines
A heat engine converts heat into mechanical work. It works by transferring heat from a high-temperature source to a low-temperature sink, performing work in the process.
Efficiency of a Heat Engine
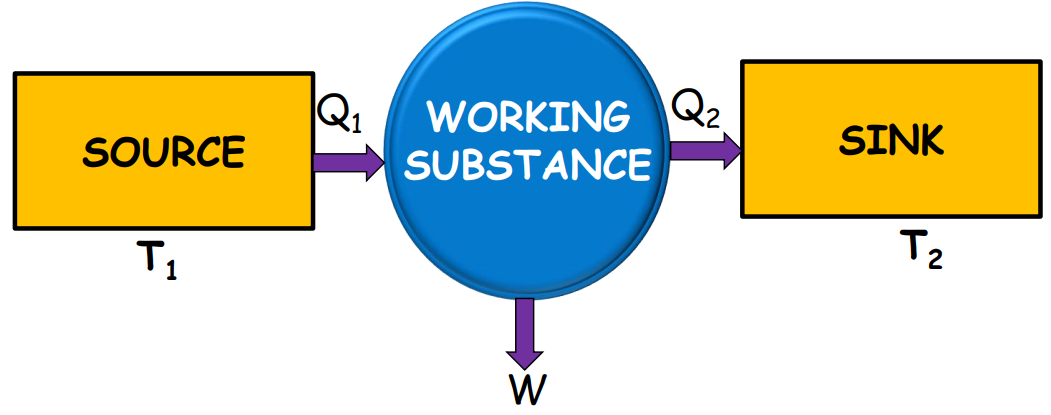
The efficiency η of a heat engine is defined as the ratio of the work done to the heat absorbed: η=W/Q1
W=Q1−Q2 i.e. η=1- (Q1/Q2)
Refrigerator
The refrigerator is the reverse of a heat engine. It extracts heat from a cold reservoir, using external work, and releases it to a hotter reservoir. The refrigeration cycle ensures that substances stay cool by constantly moving heat away.
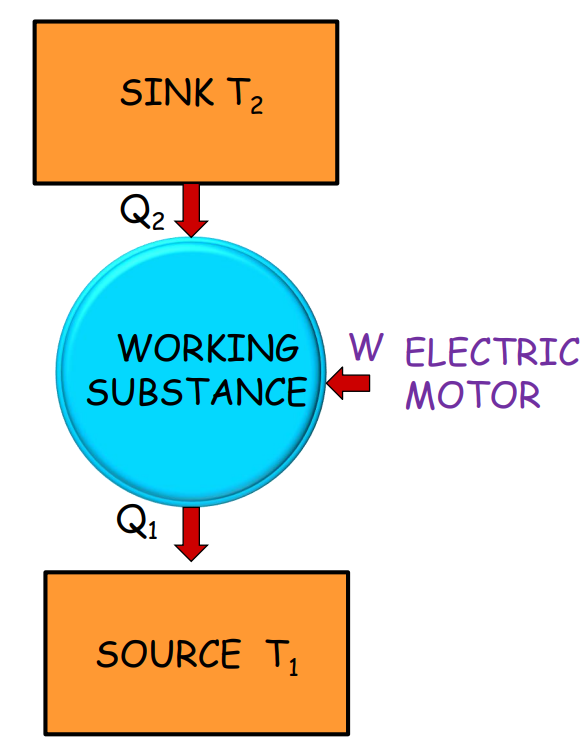
Refrigerator Components Description Sink (T2) Cold reservoir Source (T1) Hot reservoir Work (W) External work input
Second Law of Thermodynamics
The Second Law of Thermodynamics introduces the concept of irreversibility and entropy:
- Kelvin-Planck Statement: No heat engine can convert 100% of absorbed heat into work.
- Clausius Statement: It is impossible to transfer heat from a cold body to a hot body without external work.
Reversible and Irreversible Processes
- Reversible Processes: These are ideal processes where the system and surroundings can return to their original states with no changes elsewhere.
- Irreversible Processes: These processes involve natural spontaneous changes, such as heat flow from a hot to a cold body or the diffusion of gases.
Carnot Engine
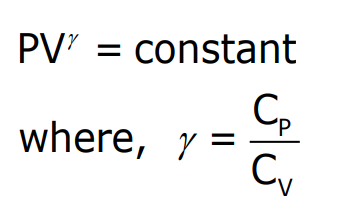
The Carnot engine represents an ideal reversible heat engine that operates between two temperatures. The Carnot cycle includes:
- Isothermal expansion
- Adiabatic expansion
- Isothermal compression
- Adiabatic compression
The Carnot Theorem states that no engine can be more efficient than a Carnot engine operating between the same two temperatures.
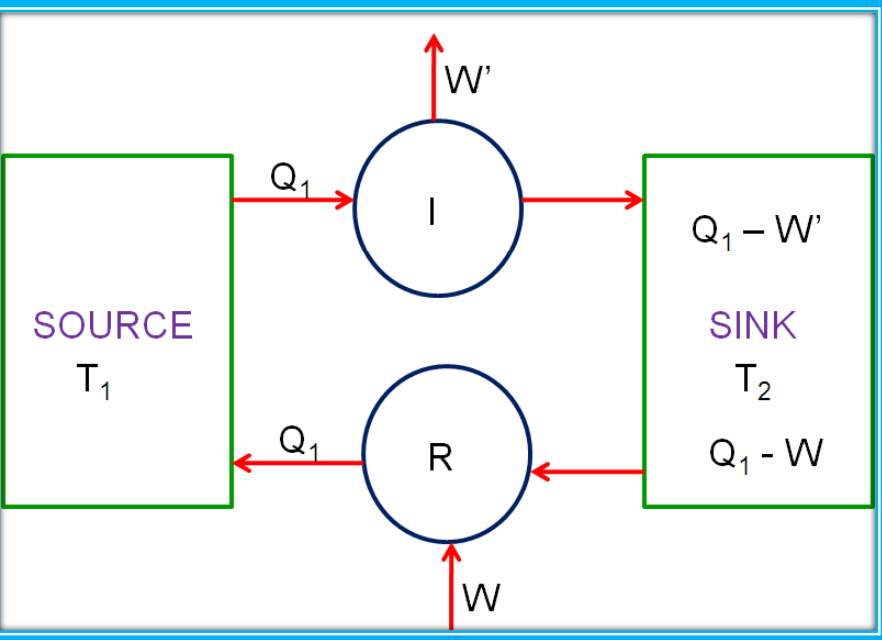
Efficiency of Carnot Engine
The efficiency of a Carnot engine is η=W/Q1 or η=1−(T2T1) Where T1 is the temperature of the heat source and T2 is the temperature of the heat sink.
Conclusion
This comprehensive guide on “Thermodynamics” provides an in-depth exploration of the fundamental aspects of physics as outlined in Class 11. It covers the core concepts of units, measurements, and the importance of standardization in scientific studies. The guide explains the laws governing heat, energy, and work, highlighting their interconnections. It delves into various thermodynamic processes like isothermal, adiabatic, and isochoric processes, explaining how they affect systems. Additionally, it introduces key concepts such as heat engines, refrigerators, and the Carnot cycle, demonstrating their practical applications in real-world systems.
Practice questions on Chapter 12 - Thermodynamics
Get your free Chapter 12 - Thermodynamics practice quiz of 20+ questions & detailed solutions
Practice Now








