Thermal Properties of Matter – Complete guide For Class 11 Physics Chapter 11
Welcome to iPrep, your Learning Super App. Our learning resources for Chapter 11, “Thermal Properties of Matter,” in Class 11 Physics are meticulously designed to ensure students gain a comprehensive understanding of this essential topic. These resources include detailed notes on heat transfer, temperature scales, and the principles of thermal expansion. We also cover the anomalous behavior of water, gas laws, and real-life applications of thermal properties in everyday phenomena. With illustrative diagrams, solved examples, and comparative tables, these resources help clarify complex concepts. Designed for in-depth learning, they serve as a crucial aid in mastering the subject.
What Are the Thermal Properties of Matter?
The concept of “Thermal Properties of Matter” in Class 11 Physics delves into the foundational principles of science by exploring the methods and standards used to quantify and describe physical phenomena. This chapter “Thermal Properties of Matter” introduces students to the behavior of materials under varying thermal conditions, emphasizing heat transfer, temperature measurement, and thermal expansion. It explains the relationship between heat and temperature and highlights the anomalous behavior of substances like water. Additionally, students learn about the practical applications of these concepts in real-world scenarios, making the chapter “Thermal Properties of Matter” both theoretically enriching and practically relevant.
What is Heat?
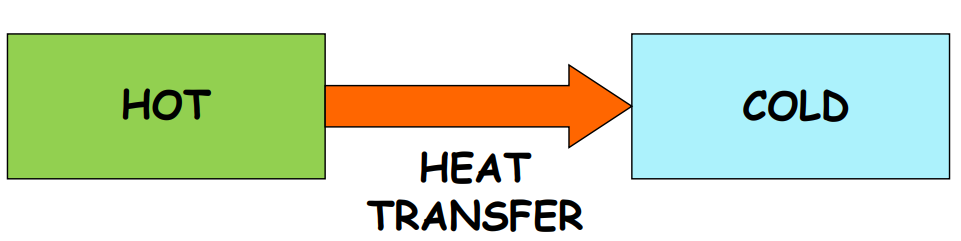
When you touch a hot frying pan, it feels hot, while an ice cube feels cold. This sensation is due to heat, a form of energy transferred between systems due to temperature differences. Heat always flows from a higher temperature to a lower temperature.

Temperature: The Indicator of Heat Flow
Temperature determines the direction of heat flow when two bodies are in contact. It is the thermal condition of a body and acts as an indicator for heat transfer.
Heat vs. Temperature
| Heat | Temperature |
| Heat is energy in transit. | Temperature measures the degree of hotness or coldness of a body. |
| Heat causes changes, while temperature reflects them. | Temperature is the effect of heat. |
Measuring Temperature: Thermometers
Temperature is measured using devices called thermometers. The most common type is the liquid-in-gas thermometer, which uses the expansion of liquids like mercury or alcohol to measure temperature.

Calibration of Thermometers
To define standard temperature scales, two reference points are commonly used:
- The freezing point of water
- The boiling point of water at standard pressure
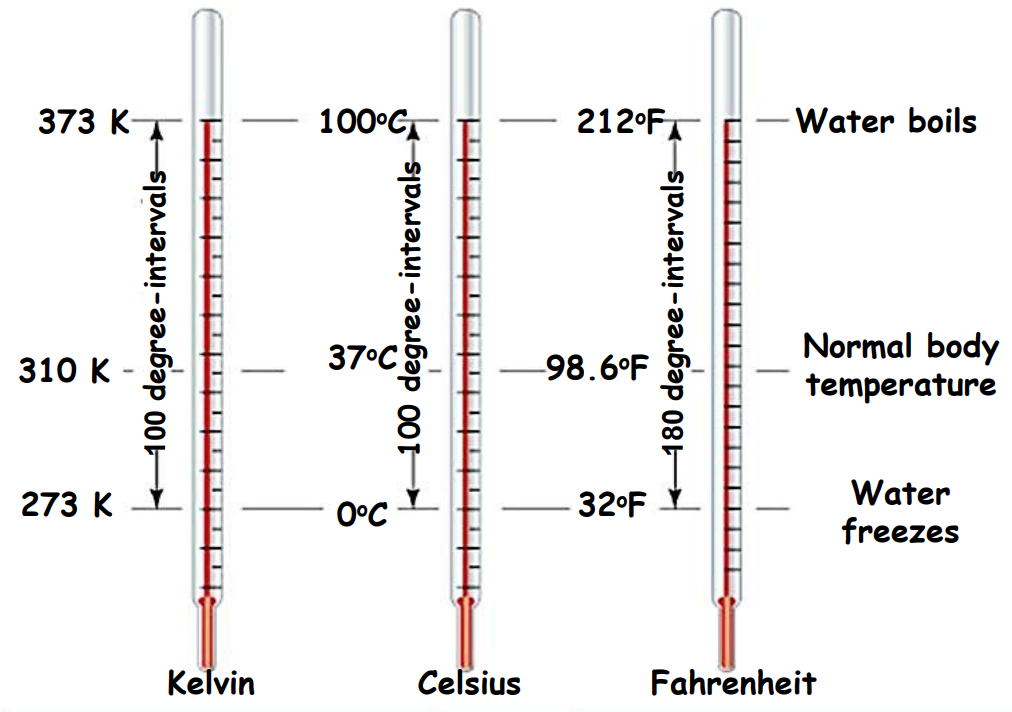
Temperature Scales: Celsius, Fahrenheit, and Kelvin
- Celsius Scale (°C):
- 0°C = freezing point of water
- 100°C = boiling point of water
- It is divided into 100 equal intervals between these points.
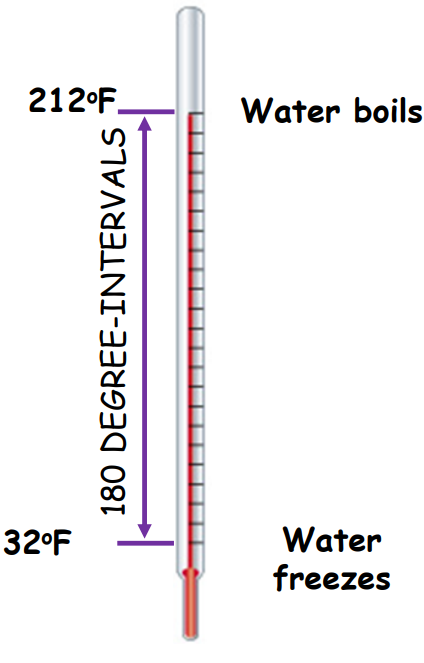
- Fahrenheit Scale (°F):
- 32°F = freezing point of water
- 212°F = boiling point of water
- The interval is divided into 180 equal parts.
- Kelvin Scale (K):
- SI unit of temperature
- Absolute zero is set at -273.15°C.
Conversion Formula:
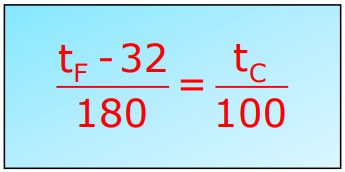
Comparison of Celsius and Fahrenheit Scale
Thermal Expansion: Solids
When a solid’s temperature increases, its molecules vibrate more, this, in turn, results in an increase in the dimensions of the body. This increase in the dimension is known as the Thermal Expansion. You can loosen a metal jar lid by running hot water over it.

Types of Thermal Expansion:
- Linear Expansion: Expansion in length.
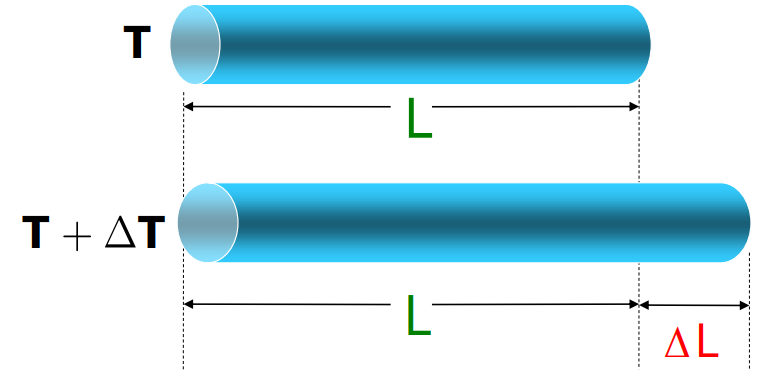
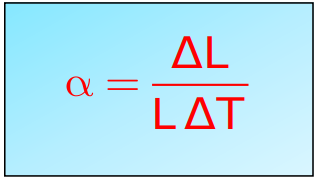
Coefficient of Linear Expansion: It is the fractional change in length per degree change in temperature. It is represented by
- Area Expansion: Increase in surface area.
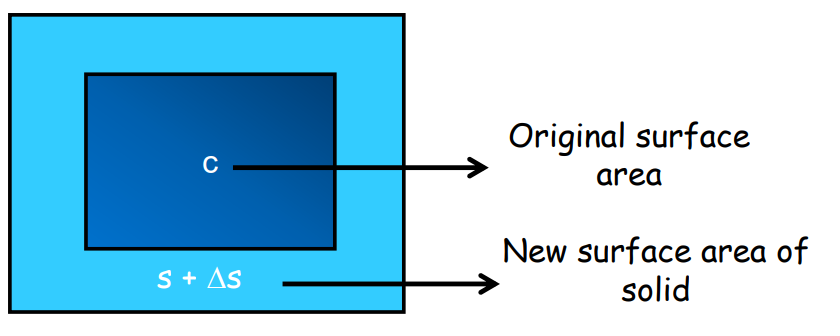
It is represented as:
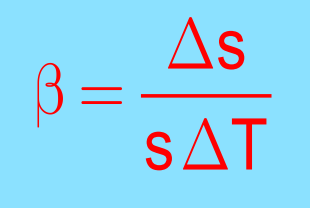
- Volume Expansion: Increase in the volume of the solid. It is represented by
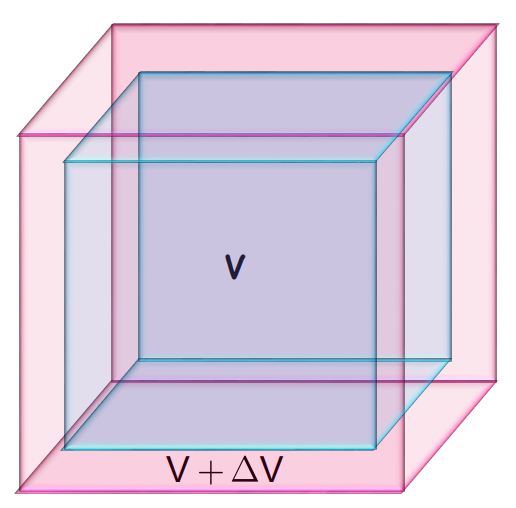
It is represented as:
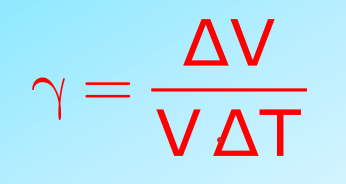
Thermal Expansion of Solids: Examples
- A metal jar lid can be loosened by running hot water over it, causing the metal to expand.
Coefficient of Expansion
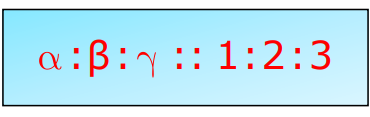
- Linear Expansion (α): Fractional change in length per degree change in temperature.
- Area Expansion (β): Fractional change in area.
- Volume Expansion (γ): Fractional volume change.
Relationship Between Coefficients:
β=2α and γ=3α
Thermal Stress
When a material is prevented from expanding or contracting due to temperature changes, thermal stress develops.
For example, a rod that is rigidly fixed at both ends will develop compressive stress if the temperature increases.
Thermal Stress Formula:
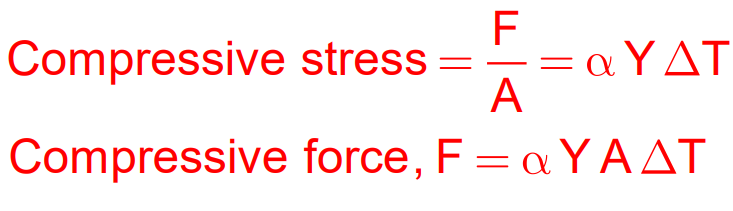
Stress=YαΔT Where:
- Y = Young’s Modulus
- α = Coefficient of Linear Expansion
- ΔT = Temperature change
- F = Force
- A = Area
Applications of Thermal Expansion
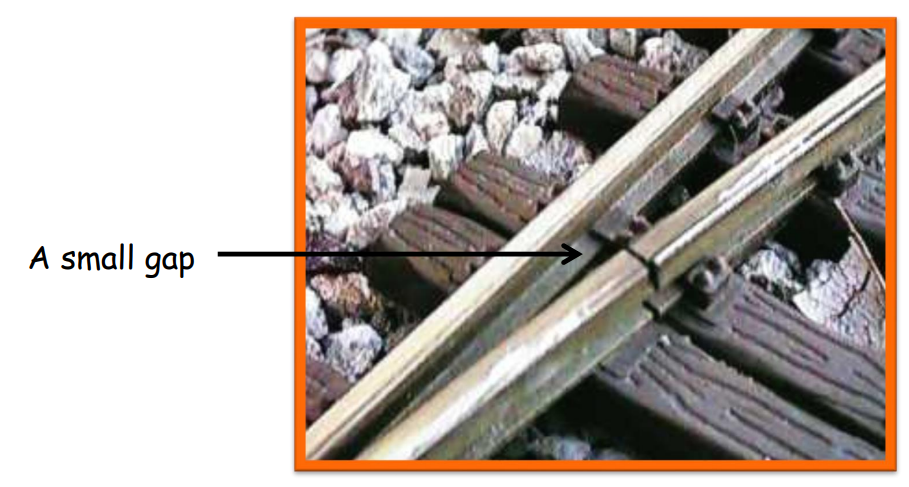
- Railway Tracks: Small gaps are left between rails to allow expansion during hot weather.
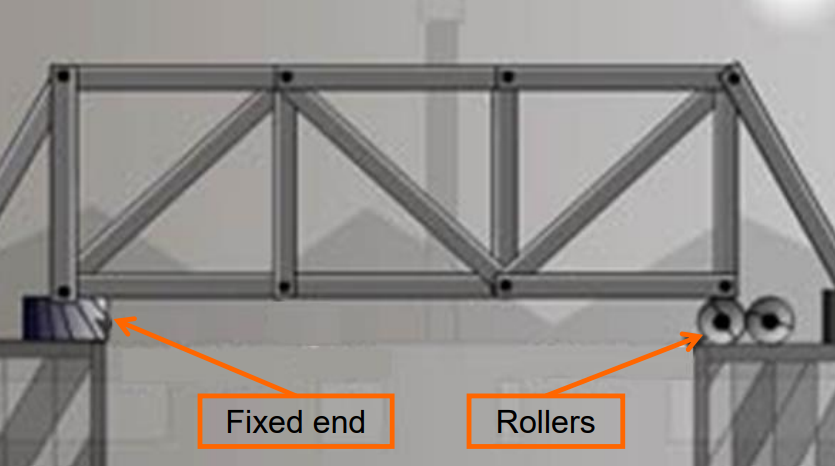
- Steel Bridges: One end is fixed, while the other rests on rollers to accommodate expansion.
Anomalous Expansion of Water
Water behaves differently from most substances. When cooled below 4°C, instead of continuing to contract, it begins to expand. This unique behavior is why water pipes burst in winter.

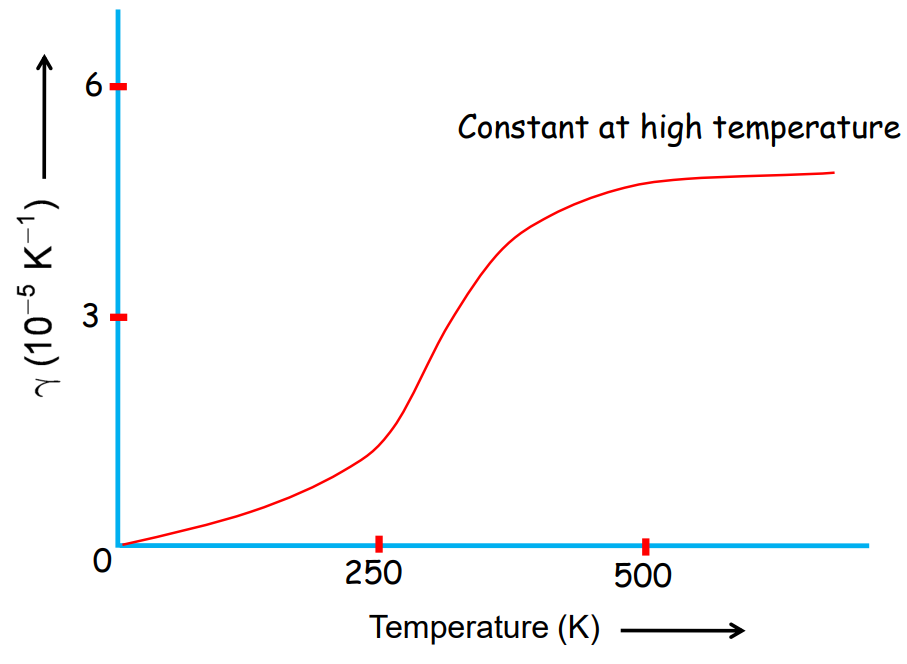
Thermal Expansion of Gases
Gases expand more than solids and liquids when heated. The coefficient of volume expansion γ\gammaγ for gases is more dependent on temperature.
For an ideal gas:
PV=RT
Where:
- P = Pressure
- V = Volume
- T = Temperature
- R = Universal Gas Constant
Heat Capacity
Heat Capacity (S) is the amount of heat required to change the temperature of a body. It is measured in Joules per Kelvin (J/K).
S=ΔQ/ΔT
Specific Heat Capacity
The heat capacity per unit mass of a substance. Its SI unit is J/kg K.
S=1/m(ΔQ/ΔT)
Where:
- m = mass
- ΔQ = heat energy
- ΔT = temperature change
Molar Specific Heat Capacity
Defined as the heat capacity per mole of a substance. The SI unit is J/mol K.
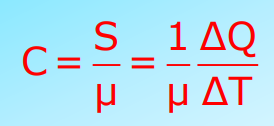
At Constant Pressure and Volume:
- Cp: Molar specific heat capacity at constant pressure.
- Cv: Molar specific heat capacity at constant volume.
Water as a Cooling Agent
- Water, with its high specific heat, is an efficient cooling agent.
- This means that a small amount of water can absorb a large amount of heat with a relatively minor rise in temperature.
- Because of this property, water is widely used in cooling systems for automobiles and engines.
- If a liquid with lower specific heat were used, its temperature would rise significantly for the same heat absorption, making water the preferred choice.
Calorimetry
Calorimetry is the science that deals with the measurement of heat. According to the principle of heat exchange, when a hot body comes in contact with a cooler one, the heat lost by the hot body is equal to the heat gained by the cooler body, assuming no heat escapes to the surroundings.
Calorimeter
A calorimeter is a device used to measure heat. It consists of a metallic vessel, typically made of copper or aluminum, along with a stirrer and an insulated lid. The vessel is well-insulated, often enclosed in a wooden jacket filled with glass wool to minimize heat loss.
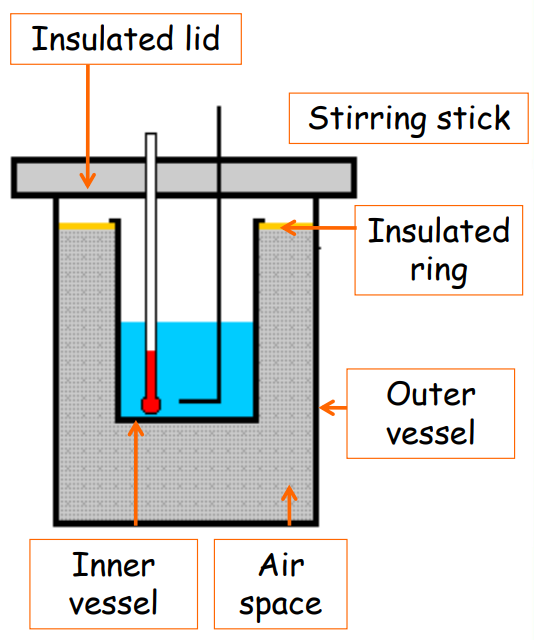
Part Description Insulated Lid Reduces heat loss Stirring Stick Helps mix the contents uniformly Outer Jacket Prevents heat exchange with surroundings Inner Vessel Holds the substance being studied Mercury Thermometer Measures temperature change
Law of Heat Exchange
The law of heat exchange can be used to calculate specific heat:
- Heat lost by body A = Heat gained by body B
Given two bodies, A and B:
- Body A: Mass mA, specific heat SA, temperature TA
- Body B: Mass mB, specific heat sB, temperature TB
- Heat will flow from A to B until both reach a common temperature, T.
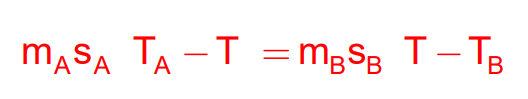
Change of State
Matter exists in three states: solid, liquid, and gas. When transitioning from one state to another, the temperature remains constant. Common state changes include:
- Solid to liquid (Melting)
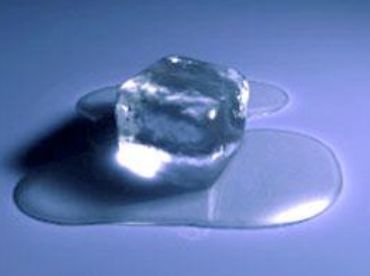
- Liquid to gas (Vaporization)

During these processes, the substance absorbs or releases heat, but its temperature doesn’t change until the entire material has transformed.
Melting Point
The melting point is the temperature at which a solid and its liquid state are in thermal equilibrium. At standard atmospheric pressure, this is known as the normal melting point.
Regelation
Regelation refers to the melting of ice under pressure and its re-solidification when the pressure is removed. A common demonstration involves placing a weighted wire on an ice block. The pressure from the wire causes the ice to melt, allowing the wire to pass through without breaking the ice block.

Vaporization and Boiling Point
- Vaporization is the transformation of a liquid to a gas.
- The boiling point is the temperature at which a liquid and gas coexist in equilibrium. As pressure increases, the boiling point rises, and vice versa.
Sublimation
Sublimation is the direct conversion of a solid into a gas without passing through the liquid phase. Examples include camphor and iodine.

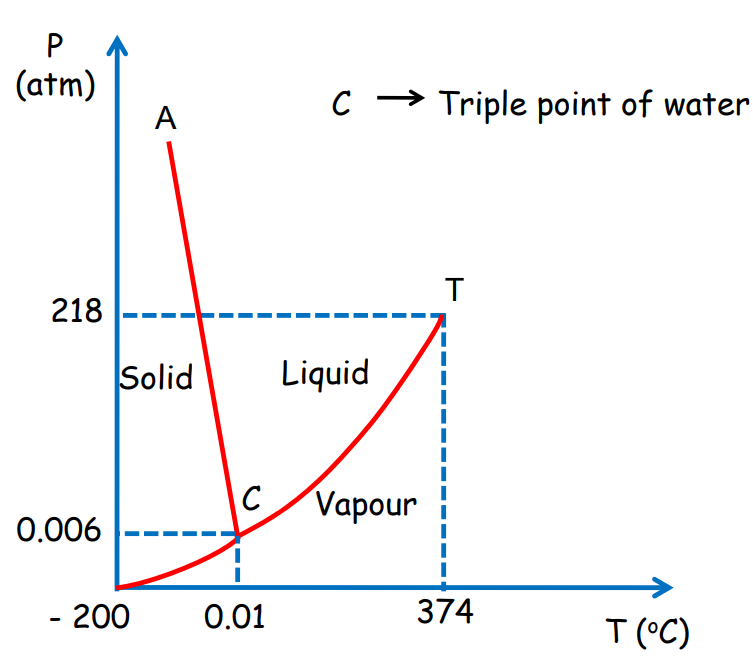
Triple Point
The triple point is the specific temperature and pressure where all three states (solid, liquid, and gas) of a substance coexist in equilibrium. For water, the triple point is 273.16 K at 6.11×10^-3 Pa.
Latent Heat
Latent heat is the amount of heat required for a substance to change its state without changing its temperature. It is defined as:
L=Q/m
Where Q is the heat energy and m is the mass. There are two types of latent heat:
- Latent heat of fusion (Lf) for solid-liquid transitions.
- Latent heat of vaporization (Lv) for liquid-gas transitions.
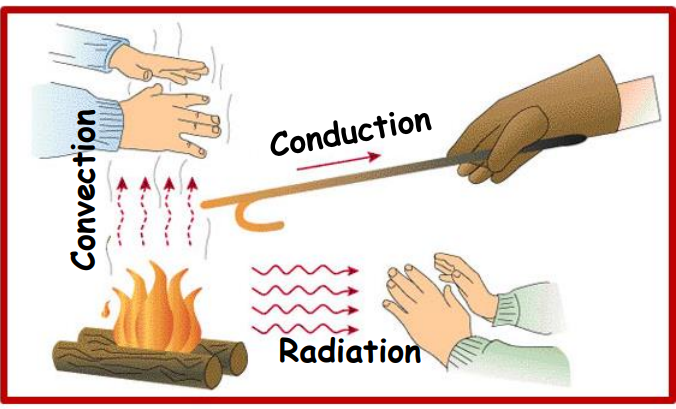
Heat Transfer
There are three modes of heat transfer:
- Conduction: Transfer of heat through a material by direct contact.
- Convection: Transfer of heat in fluids (liquids or gases) through fluid motion.
- Radiation: Transfer of heat through electromagnetic waves without requiring a medium.
Conduction
In conduction, heat flows through a material due to temperature differences between adjacent parts. A classic example is heating one end of a metal rod; the other end gradually becomes hot due to conduction.
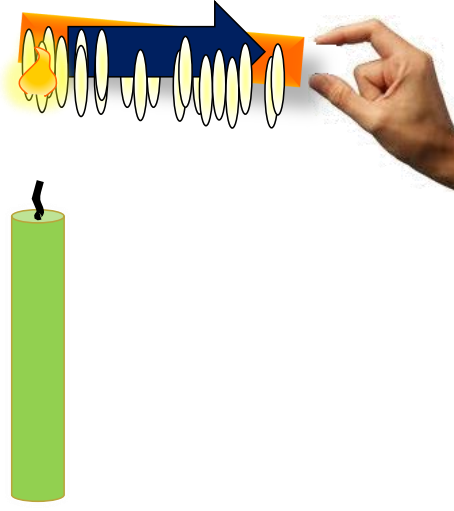
In a steady state, no part of the conductor absorbs heat, while in a variable state, different sections heat up over time.
Thermal Conductivity
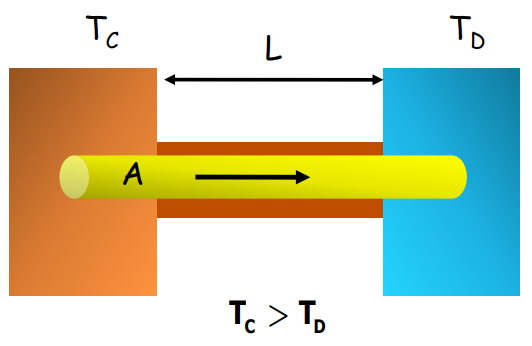
The rate at which heat flows through a material is determined by its thermal conductivity, K. This is given by:

Where:
- H is the rate of heat flow
- A is the cross-sectional area
- L is the length of the conductor
- TC and TD are temperatures at either end
Convection
Convection is the transfer of heat within a fluid (liquids and gases) from regions of higher temperature to lower temperature, aided by the movement of the fluid itself. Let’s explore how this process works:
Natural Convection in Liquids
- When the temperature of the lower region in a fluid is higher than that of the upper region, the density of the lower region decreases.
- Buoyancy causes the warmer, less dense fluid to rise while cooler fluid particles from the upper region descend to take their place. This cycle continues until the temperature becomes uniform throughout the fluid.
Natural Convection in Air
- During the day, the ground heats faster than water bodies, causing the air in contact with the ground to heat up, expand, and rise.
- Cooler air replaces this warm air, creating air currents that circulate. At night, this process reverses as the ground cools faster than water.
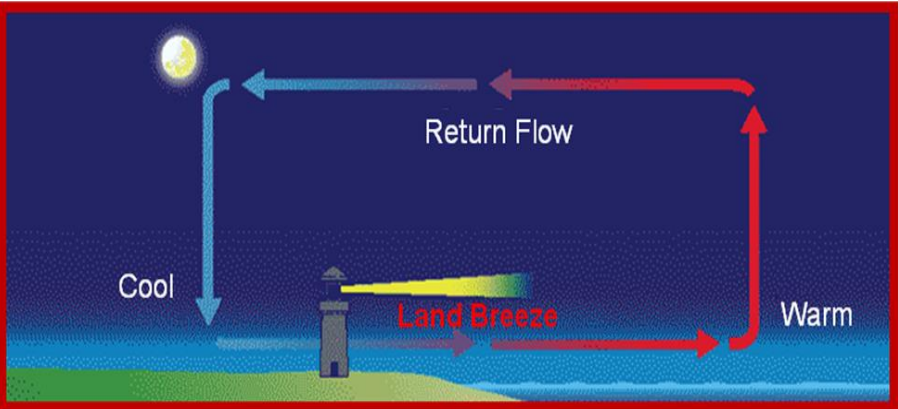
Sea and Land Breezes
- Sea Breeze (Daytime): The land heats up faster than the sea, and the warmer air from land moves towards the sea, while cooler air from the sea moves inland to take its place.
- Land Breeze (Nighttime): Water retains its heat longer than land, so warmer air from the sea moves towards land, while cooler air from land moves towards the sea.
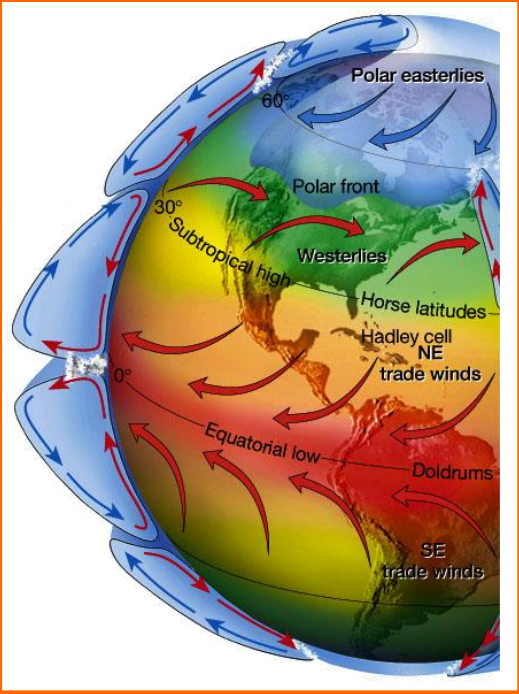
Trade Winds
- Trade winds are steady surface winds that blow from the northeast towards the equator. This happens because the equatorial region receives more solar heat than the polar regions, setting up a convection current.
- Due to Earth’s rotation, air descends at about 30° N latitude and returns to the equator.
Forced Convection
In forced convection, the movement of the fluid is driven by external forces such as pumps or fans. For example, the air heating systems used in homes work on the principle of forced convection.
Radiation
Radiation is the transfer of heat through electromagnetic waves, requiring no medium. Solar energy is a prime example of radiation.
- Heat energy travels at the speed of light (3 x 10^8 m/s).
- When thermal radiation falls on an object, part of it is reflected while the rest is absorbed. The amount of heat absorbed depends on the color of the body.
- Black surfaces absorb and emit radiant energy better than lighter-colored ones. That’s why we prefer to wear light-colored clothes in summer and darker ones in winter.
Practical Uses of Radiation
- Blackened cooking utensils absorb maximum heat, helping cook food efficiently.
- We see Newton’s law of cooling apply when objects release heat at a rate proportional to the temperature difference between the object and its surroundings.
Newton’s Law of Cooling
Newton’s Law of Cooling states that the rate of heat loss from a body is directly proportional to the temperature difference between the body and its surroundings, provided the difference is small. The rate of heat loss also depends on the surface area and nature of the body.
The mathematical expression is:
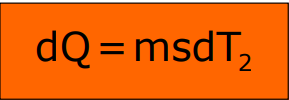
Where T2 is the body temperature, T is the surrounding temperature, and k is a constant.
Conclusion
This comprehensive guide on “Thermal Properties of Matter” provides an in-depth exploration of the fundamental aspects of physics as outlined in Class 11. It covers core concepts of the transfer of heat, temperature scales, and the expansion of solids, liquids, and gases under varying thermal conditions. Students are introduced to key ideas like specific heat capacity, thermal stresses, and the anomalous expansion of water. The guide also dives into the practical applications of thermal properties, from everyday occurrences to engineering solutions, ensuring a thorough grasp of both theoretical knowledge and its real-world relevance.
Practice questions on Chapter 11 - Thermal Properties of Matter
Get your free Chapter 11 - Thermal Properties of Matter practice quiz of 20+ questions & detailed solutions
Practice Now