Units And Measurements – Complete Guide For Class 11 Physics Chapter 1
Welcome to iPrep, your Learning Super App. Our learning resources for Chapter 1, “Units and Measurements,” in Class 11 Physics are meticulously designed to ensure students gain a comprehensive understanding of this essential topic. These resources include detailed notes on fundamental concepts such as physical quantities, the International System of Units (SI), and dimensional analysis. Additionally, they provide comprehensive explanations of significant figures, accuracy, and precision in measurements, helping students grasp the importance of reliable data in scientific studies. The resources also cover methods of error estimation and error propagation to enhance students’ problem-solving skills. With ample practice questions and examples, these materials are crafted to build a strong foundation for future physics topics.
What Are Units And Measurements?
The concept of “Units and Measurements” in Class 11 Physics delves into the foundational principles of science by exploring the methods and standards used to quantify and describe physical phenomena. This chapter introduces students to the International System of Units (SI), the standard set of units for scientific measurements, ensuring uniformity and consistency across the globe. It covers various physical quantities, their dimensional formulas, and the importance of dimensional analysis in understanding relationships between different quantities. Additionally, it emphasizes the role of accuracy, precision, and significant figures in measurements, highlighting the necessity of careful data collection and interpretation in scientific research. Understanding these concepts of units and measurements is crucial for students to effectively engage with more complex topics in physics and other scientific disciplines.
Systems of Units
Historical Systems of Units
Over time, scientists have used various systems of units:
- CGS System: Centimeter (length), Gram (mass), Second (time)
- FPS System: Foot (length), Pound (mass), Second (time)

- MKS System: Meter (length), Kilogram (mass), Second (time)
International System of Units (SI)
As mentioned in the chapter units and measurements, the International System of Units (SI) is the globally accepted system used by scientists today. It consists of seven fundamental units, with two additional supplementary units for measuring angles.
S.No. Physical Quantity Fundamental Unit Symbol 1 Mass Kilogram kg 2 Length Meter m 3 Time Second s 4 Temperature Kelvin K 5 Electric Current Ampere A 6 Luminous Intensity Candela cd 7 Quantity of Matter Mole mol
Supplementary Units:
S.No. Supplementary Physical Quantity Supplementary Unit Symbol 1 Plane Angle Radian rad 2 Solid Angle Steradian sr
Definitions of Fundamental Units
According to the Units and Measurements Chapter, Each fundamental unit has a precise definition, which ensures consistency in measurements across the globe:
- Meter: Length of the path covered by light in vacuum in 1/299,792,458 of a second.
- Kilogram: Mass of the international prototype of the kilogram, a platinum-iridium alloy block stored at Sevres, France.
- Second: Duration of 9,192,631,770 periods of radiation corresponding to the transition between two hyperfine levels of the ground state of Cs-133 atom.
- Ampere: Constant current which, when maintained in two straight parallel conductors of infinite length, placed 1 meter apart in vacuum, produces a force of 2 × 10⁻⁷ N/m.
- Kelvin: Fraction of 1/273.16 of the thermodynamic temperature of the triple point of water.
- Candela: Luminous intensity of a source emitting monochromatic radiation at a frequency of 540 × 10¹² Hz with a radiant intensity of 1/683 watt per steradian.
- Mole: Amount of substance containing as many elementary entities as atoms in 0.012 kg of carbon-12.

Supplementary Units
- Radian: Angle subtended at the center of a circle by an arc equal in length to the radius of the circle.
- Steradian: Solid angle subtended at the center of a sphere by a surface area equal to the square of the sphere’s radius.
Derived Units
Derived units are measurements obtained by combining fundamental units. For example:
- Speed: Derived from the fundamental units of length and time.
- Density, Acceleration, Energy, Pressure: Derived from various combinations of fundamental units.
Measurement of Length
Lengths encountered in everyday life can vary significantly, from the size of a molecule to the distance between planets. There are two primary methods for measuring length:
Direct Method
- Meter Scale: For measuring lengths ranging from 10⁻³ m to 10² m.
- Vernier Calipers: For lengths of the order of 10⁻⁴ m.
- Screw Gauge and Spherometer: For lengths of the order of 10⁻⁵ m.

Indirect Method
- Parallax Method: Used to determine distances of stars less than 100 light years away and the distance between the Earth and the Moon. The method is based on observing an object’s apparent change in position when viewed from two different points.
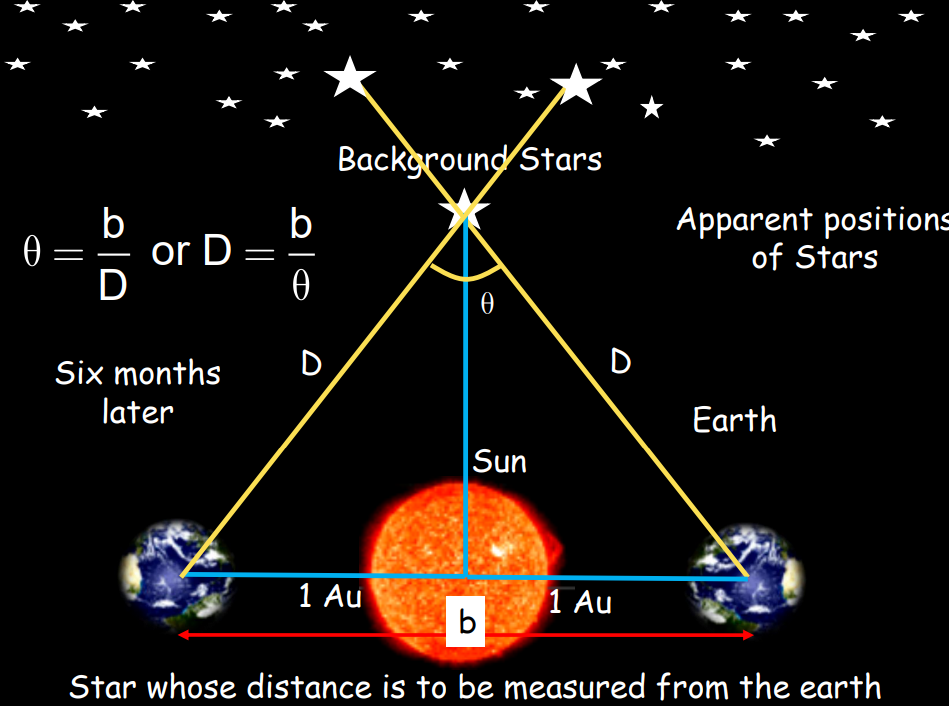

Estimation of Very Small Distances
For determining molecular sizes (order of 10⁻⁸ m to 10⁻¹⁰ m), special methods and optical microscopes are used. For sizes smaller than 10⁻¹⁰ m, electron beams are employed.
Range of Lengths
- 1 Fermi = 1 f = 10⁻¹⁵ m
- 1 Angstrom = 1 Å = 10⁻¹⁰ m
- 1 Astronomical Unit (AU) = 1.496 × 10¹¹ m
- 1 Light Year = 9.46 × 10¹⁵ m
- 1 Parsec = 3.08 × 10¹⁶ m
Measurement of Mass
Mass is a fundamental property of matter and is independent of external factors like temperature or location. The SI unit of mass is the kilogram (kg).

Measurement Techniques
- Common Balances: Used for commonly available masses.
- Gravitational Method: Used for measuring the mass of large objects like planets and stars.
Mass of an Atom
The Unified Atomic Mass Unit (u) is used for tiny particles like atoms. It is defined as 1/12th of the mass of a carbon-12 atom, which includes the mass of electrons.
Range of Masses
Masses of various objects can vary from the mass of an electron (10⁻³⁰ kg) to the mass of the known universe (10⁵⁵ kg).
Object Mass (kg) Electron 10⁻³⁰ Proton 10⁻²⁷ Rain Drop 10⁻⁶ Moon 10²³ Earth 10²⁵ Sun 10³⁰ Milky Way 10⁴¹
Measurement of Time
The Atomic Standard of Time is used for precise time measurement. The SI unit of time is the second, defined as the time interval for 9,192,631,770 vibrations of radiation between the two hyperfine levels of the cesium-133 atom.
Atomic Clocks
Atomic clocks are the most accurate timekeeping devices, with uncertainties as low as 1 part in 10¹³.
Accuracy and Precision
Accuracy refers to how close a measured value is to the true value, while precision refers to the resolution or limit of the measurement. The precision of a measurement depends on the accuracy of the instrument used.
Understanding Accuracy and Precision
For example, if the true length of a pencil is 5.437 cm, an instrument with 0.1 cm resolution might measure it as 5.3 cm, while one with 0.01 cm resolution might measure it as 5.26 cm. The first measurement is more accurate, but the second is more precise.
Errors in Measurements
Errors are uncertainties in measurements and can be classified into:
- Systematic Errors: Occur in one direction (positive or negative) due to factors like instrument imperfections, experimental technique flaws, or personal biases.
- Random Errors: Occur irregularly due to unpredictable fluctuations in experimental conditions or unbiased personal errors.
Systematic Errors
- Instrumental Errors: Caused by design flaws or calibration issues.
- Experimental Technique Errors: Due to improper methods or external conditions like temperature or humidity changes.
- Personal Errors: Due to individual biases or carelessness.
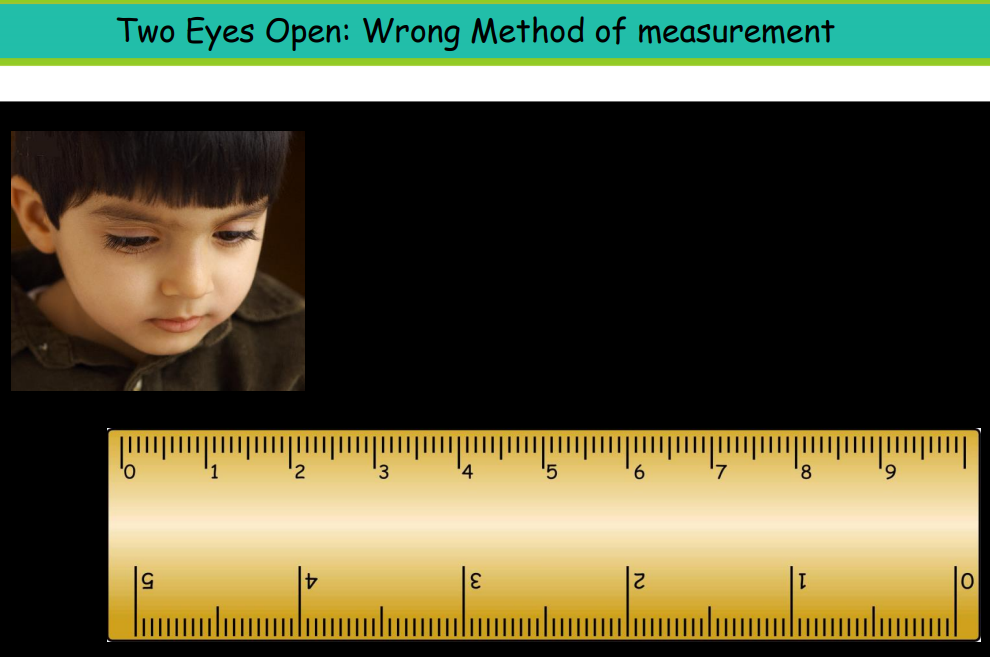
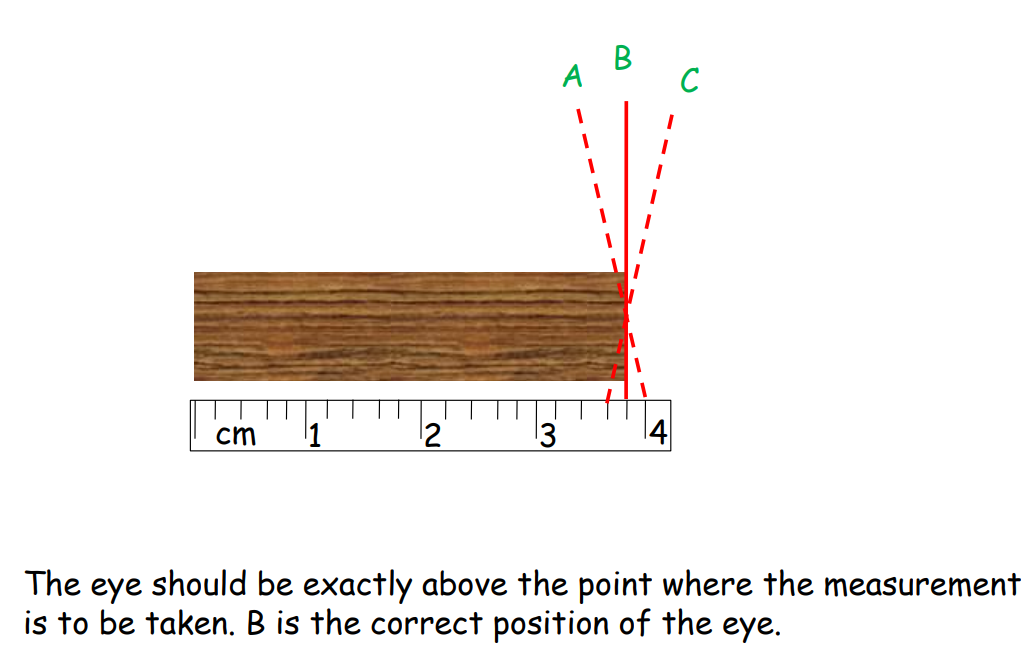
Random Errors
These occur randomly and can be reduced by repeated measurements.
Least Count
The least count is the smallest value measured by an instrument. It is associated with the resolution of the instrument and is considered a type of random error.
Absolute, Relative, and Percentage Errors
- Absolute Error: Difference between the experimental value and the true value.
- Relative Error: Ratio of the mean absolute error to the mean value of the quantity.
- Percentage Error: Relative error expressed as a percentage.
Combination of Errors
When combining measurements:
- Sum/Difference: Absolute errors add up.
- Product/Quotient: Relative errors add up.
- Powers: The relative error is multiplied by the exponent.
Significant Figures
As per the chapter units and measurements, significant figures are digits in a measurement that are known reliably plus one uncertain digit. They indicate the precision of the measurement and should not be more than the precision of the instrument used.
Determination of Significant Figures
- All non-zero digits are significant.
- Zeros between non-zero digits are significant.
- Leading zeros in decimal numbers are not significant.
- Terminal zeros in whole numbers are not significant unless specified by a decimal point.
Rounding Off
When rounding off, follow these rules:
- If the digit to be dropped is more than 5, round up.
- If it is less than 5, round down.
- If it is 5, round to make
Conclusion
This comprehensive guide on “Units and Measurements” provides an in-depth exploration of the fundamental aspects of physics as outlined in Class 11. It covers the core concepts of units, measurements, and the importance of standardization in scientific studies. The guide explains different types of measurement units, including base units and derived units, and how these are used to quantify physical quantities. It delves into the significance of measurement accuracy and precision, along with the role of significant figures in reporting data. Furthermore, the guide discusses dimensional analysis, a powerful tool for checking the consistency of equations and deriving relationships between various physical quantities, which is essential for problem-solving in physics.
Practice questions on Chapter 1 - Units And Measurements
Get your free Chapter 1 - Units And Measurements practice quiz of 20+ questions & detailed solutions
Practice Now








